Research Article
Analysis of Factors Influencing the Prognosis of Neoadjuvant Chemotherapy for Breast Cancer with Peripheral Blood Inflammatory Markers 
2 Department of radiology, the first hospital of Handan city, Hebei, China
3 Department of ultrasound, the first hospital of Handan city, Hebei, China
4 Department of neurology, the first hospital of handan city, Hebei, China
 Author
Author  Correspondence author
Correspondence author
Cancer Genetics and Epigenetics, 2020, Vol. 8, No. 1 doi: 10.5376/cge.2020.08.0001
Received: 27 Oct., 2020 Accepted: 18 Nov., 2020 Published: 27 Nov., 2020
Liu S.H., Zhang X., Hu G.Q., and Cui R.N., 2020, Analysis of factors influencing the prognosis of neoadjuvant chemotherapy for breast cancer with peripheral blood inflammatory markers, Cancer Genetics and Epigenetics, 8(1): 1-8 (doi: 10.5376/cge.2020.08.0001)
To explore the factors influencing the prognosis of neoadjuvant chemotherapy for breast cancer. From January 2014 to January 2017 123 breast cancer patients receiving neoadjuvant chemotherapy (NAC) were selected, establish the optimal threshold value of peripheral blood inflammation indicators, patients were divided into high and low groups according to the optimal threshold, contrast different peripheral blood inflammation indexes with pathological complete remission (pCR) after chemotherapy, relationship between disease-free survival (DFS). Patients were divided into two groups with high and low ratios according to the critical values of neutrophil/lymphocyte ratio (NLR) 2.34 and PLR critical value 130.21, the pCR rate of patients with low NLR and PLR group was significantly higher than that of patients with high NLR and high PLR group (P=0.001), patients with high NLR had shorter DFS than those with low NLR (P=0.001), patients with high PLR had shorter DFS than those with low PLR (P=0.001). In patients without pCR after NAC, the DFS of patients in the high NLR group was worse than that in the low NLR group, DFS of patients in the high NLR group were worse than those in the low NLR group, DFS of patients with high PLR group was also worse than that of patients with low PLR group (All P<0.05). Multivariate analysis showed that high PLR and KI-67 were the factors that affected the poor prognosis of breast cancer patients who had received NAC, high NLR was not an independent prognostic factor. High levels of NLR and PLR in peripheral blood before NAC predict poor prognosis in breast cancer patients, Ki-67 and PLR are independent risk factors.
Breast cancer is the most common malignancy in women today, it ranks first in the incidence of female malignant tumors (Ngui et al., 2016). Neoadjuvant chemotherapy has now become the standard of care for locally advanced breast cancer and breast cancer patients who expect to keep their breast in a healthy state, patients who achieved pathological complete response after neoadjuvant chemotherapy had a longer disease-free survival time, which predicted a better prognosis (Feliciano et al., 2017). At present, clinical stage, lymph node metastasis status, tumor size, hormone receptor expression and other indicators are often used to evaluate the prognosis, it has important guiding significance to develop the whole course management strategy for breast cancer patients (Zhao et al., 2019) Studies show that (Michaud et al., 2015; Chenet et al., 2018) the biological behavior of breast cancer is closely related to the activation of inflammatory response, neutrophil to lymphocyte ratio (NLR) Platelet to lymphocyte ratio (PLR) is a sensitive indicator of the inflammatory state of the body, it has predictive value for the efficacy of neoadjuvant chemotherapy. The purpose of this study was to investigate the predictive value of peripheral inflammatory markers for the efficacy and prognosis of neoadjuvant chemotherapy for breast cancer.
1 Materials and Methods
1.1 General information
A total of 123 breast cancer patients receiving neoadjuvant chemotherapy admitted to six departments outside our hospital from January 2014 to January 2017 were selected, Ages 28~60, the mean age was 49.79±8.63 years, all were women, clinical stage II-III. Inclusion criteria: (1) routine blood test within 7 days before the first neoadjuvant chemotherapy, (2) patients receiving neoadjuvant chemotherapy, (3) Normal liver and kidney function. Exclusion criteria: (1) patients with other malignant tumors or diseases of the blood system, (2) Pregnancy or lactation, (3) Transfer at the far turn, (4) Suffering from acute and chronic inflammatory diseases such as cold and respiratory tract infection before the first neoadjuvant chemotherapy. This study was approved by the medical ethics committee of our hospital, all patients volunteered to participate in this study and signed informed consent before treatment.
1.2 Detection of inflammatory markers
Routine blood test results of patients 7 days before the first neoadjuvant chemotherapy were collected, these include platelet count, neutrophil count, lymphocyte count, and the ratio between the two.
1.3 Determination of immunohistochemical criteria
The expression states of ER, PR, HER-2 and Ki67 were determined by immunohistochemical sections. Positive ER and PR: The percentage of nucleus staining positive cells ≥1%. Its ehrs positive -2: The nucleus staining of > cells in 30% was +++ Her-2. Its ehrs -2 negative: Tumor nuclei were stained with 0 and +, and fluorescence in situ hybridization (FISH) was detected as without amplification. Ki67: Ki67 positive is defined as brownish yellow granule deposits in the nucleus, 10 fields were selected to calculate the percentage of Ki67 positive cells according to the total tumor cells, Ki67 expression ≥14% was defined as high expression and Ki67 expression <14% as low expression.
1.4 Efficacy evaluation and follow-up
Response evaluation criteria Solid tumors, (RECIST) 1.1 Evaluation standard was applied (Duffaud et al., 2000). Complete remission (CR): disappearance of all known lesions, Partial Response (PR): after treatment, the sum reduction of tumor maximum diameter ≥30%, Progress disease (PD): reduce the maximum tumor diameter by no more than 30% but not reach the level of PR or increase it by no more than 20% and not reach the level of PD, Stabli disease (SD): The sum of maximum diameter of lesions decreased but did not reach PR or increased but did not reach PD. NAC was clinically effective as CR+PR and ineffective as PD+SD. Pathological definition of complete remission (Liang et al., 2016): no invasive carcinoma or carcinoma in situ was found in the primary breast tumor lesions and lymph nodes after surgery.
The patients in this study were followed up by telephone, outpatient review and re-hospitalization. The starting point was the date of first diagnosis, and the end point was the date of loss of follow-up and death. The follow-up period was 2019-December. The first distant metastasis or local regional recurrence after surgery was defined as a disease-free survival (DFS) event. If no recurrence or metastasis occurred, the endpoint of follow-up or death would be the truncation time.
1.5 Statistical analysis
SPSS 25.0 statistical software was used for analysis. Measurement data were expressed as mean ± standard deviation (![]() ±s), and counting data were expressed as frequency or %, using χ2 the correlation between inflammatory markers and clinicopathological data and pCR after neoadjuvant chemotherapy was analyzed, the patient survival curve was plotted using the Kaplan-Meier method, using log-rank test to compare the survival differences of each group of variables, the single factor was incorporated into the multivariate analysis with the variable P<0.01, P<0.05 was considered statistically significant.
±s), and counting data were expressed as frequency or %, using χ2 the correlation between inflammatory markers and clinicopathological data and pCR after neoadjuvant chemotherapy was analyzed, the patient survival curve was plotted using the Kaplan-Meier method, using log-rank test to compare the survival differences of each group of variables, the single factor was incorporated into the multivariate analysis with the variable P<0.01, P<0.05 was considered statistically significant.
2 Results
2.1 Relationship between clinicopathological factors and pathological complete response after breast neoadjuvant chemotherapy
As shown in Table 1, the pCR rate of breast cancer patients after NAC was closely correlated with ER, PR, Ki67, lymph node metastasis, lymphocyte count, NLR and PLR (all P<0.05).
.png) Table 1 Relationship between clinicopathological factors and pathological complete response after breast neoadjuvant chemotherapy (n=123) |
2.2 Correlation between NLR, PLR and prognosis of patients with out pCR after NAC
According to the NLR critical value of 2.34 and PLR critical value of 130.21, patients who did not achieve pCR after surgery were divided into two groups with high and low ratio, NLR > 2.34 is the high group, NLR≤2.34 is the low group, PLR > 130.21 is the high group, PLR≤130.21 is the low group, the results showed that non-PCR patients with high NLR and PLR had a poor prognosis ( all P<0.05) (Table 2).
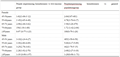 Table 2 Correlation between NLR, PLR and prognosis of patients without pCR after NAC |
2.3 Correlation between NLR and PLR and prognosis of breast cancer patients with NAC
Patients were divided into two groups according to the NLR threshold value of 2.34, NLR > 2.34 is the high group (n=78), NLR≤2.34 is low group (n=45), at the end of follow-up, the DFS of patients with high and low NLR was 29 months and 35 months, respectively, by log-rank test, the risk of recurrence was higher in the high NLR group than in the low NLR group, P=0.001 (Figure 1).
.png) Figure 1 Comparison of DFS of breast cancer patients in the high NLR group and the low NLR group |
Patients were divided into two groups according to PLR threshold value of 130.21, PLR>130.21 is the high group (n=72), PLR≤130.21 is the low group (n=51), at the end of follow-up, DFS of patients with high and low PLR was 26 months and 33 months, respectively, the risk of recurrence was higher in the PLR high group than in the PLR low group by the log-rank test, P=0.001 (Figure 2).
.png) Figure 2 Comparison of DFS curves between the HIGH PLR group and the low NLR group |
2.3 Single factor analysis of DFS of neoadjuvant chemotherapy for breast cancer
Univariate analysis of DFS affecting neoadjuvant chemotherapy for breast cancer showed that, age, platelet count, NLR, PLR, ER, PR and KI-67 may be influential factors for the prognosis of breast cancer patients receiving NAC (all P<0.05) (Table 3).
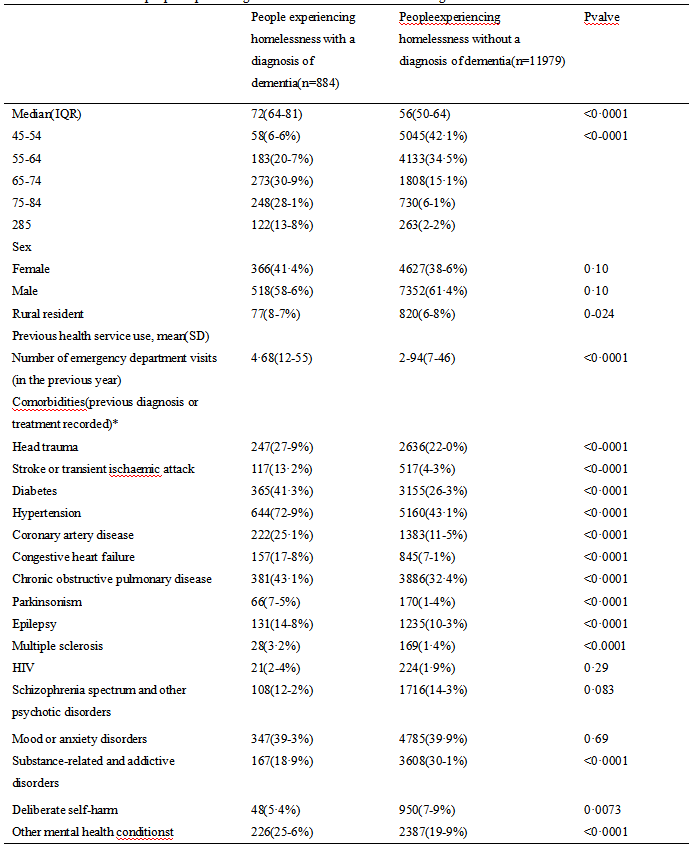 Table 3 Single factor analysis of DFS of neoadjuvant chemotherapy for breast cancer |
2.4 Multi-factor analysis of DFS of neoadjuvant chemotherapy for breast cancer
When factors in univariate analysis (P<0.05) were included in multivariate analysis, the results showed that, high PLR and KI-67 are adverse prognostic factors for breast cancer patients receiving NAC, (all P<0.05) (Table 4).
 Table 4 Multi-factor analysis of DFS of neoadjuvant chemotherapy for breast cancer |
3 Discussion
NAC is the standard therapy for patients with locally advanced and inflammatory breast cancer, can stage and degrade a tumor that is not suitable for surgical resection, and the opportunity for further breast conserving surgery, and for axillary lymph nodes from positive to negative, it also avoids axillary lymph node dissection, through the subsequent combination of treatment, to improve local control of the tumor, and then improve the prognosis. PCR after NAC is considered to be an important indicator of patients' short-term efficacy and long-term survival benefit (Brar et al., 2019), although most patients can achieve pCR after NAC treatment, but patients who did not achieve pCR had a higher risk of recurrence and a worse prognosis, therefore, it is very important to find the prognostic factors of breast cancer. At present, TNM stage, estrogen, progesterone receptor, human surface skin growth factor receptor 2 and KI-67 are recognized prognostic indicators for breast cancer patients. However, recent studies have shown that there is a close relationship between the occurrence and development of breast cancer and the inflammatory response and immune state of the body, tumor cells can release a variety of cytokines that cause non-specific inflammatory responses in the body, corresponding inflammatory cells can assist tumor cell proliferation and growth, angiogenesis, and promote the occurrence and development of tumors (Powell et al., 2017). The body's inflammatory response includes various markers such as neutrophils, lymphocytes, and platelets, Among them, neutrophils secrete a variety of cytokines, can stimulate capillary proliferation, promote tumor growth and metastasis. The decrease of lymphocytes indicates that the anti-tumor immunity of the body is reduced, providing an environment for the growth of cancer cells (Duan et al., 2018).Platelets can secrete platelet chemokine growth factor, platelet fourth factor, transforming growth factor B and VRGF to stimulate tumor differentiation and promote tumor cell proliferation (Raungkawemane et al., 2012). NLR is an indicator reflecting the ratio of neutrophils to lymphocytes, PLR is the ratio of reactive platelets to lymphocytes, NLR and PLR can stabilize the inflammatory response of the body, in the course of tumor genesis and development, there's an imbalance between the body's anti-tumor immune system and its inflammatory response, lymphocytic decline, neutrophils, platelet increase, corresponding increase in NLR and PLR. Studies have found that (Swierczak et al., 2015; Coffelt et al., 2016). NLR and PLR have predictive value for the efficacy of neoadjuvant chemotherapy for breast cancer. Asano (Asano et al., 2016). found in their research on breast cancer NAC that NLR was correlated with THE pCR rate and prognosis of triple negative breast cancer. Chen (Chen et al., 2016) analyzed NAC of 215 cases of breast cancer and showed that low NLR level before treatment (<2.06) was associated with high pCR rate after NAC treatment. Azab B (Azab et al., 2012) observed the short-term and long-term survival of 316 breast cancer patients, it was found that preoperative blood NLR > 3.3 was associated with higher 1-year and 5-year mortality rates, therefore, NLR level (>3.3) was considered as an independent predictor of short-term and long-term mortality in breast cancer patients. Gunduz S (Gunduz et al., 2015) showed that preoperative PLR > 200 suggested a longer median survival time for patients. All the above results suggest that NLR and PLR may be used as prognostic indicators, the results of this study showed that the levels of peripheral blood, NLR and PLR before treatment could predict the prognosis of breast cancer patients receiving NAC, In other words, patients with high levels of NLR and PLR have a worse prognosis. For patients without pCR after NAC, the two indexes are also of predictive significance.
In this study, low levels of NLR and PLR had relatively high pCR rates, univariate analysis showed that NLR and PLR were one of the predictors of pCR, however, in the multi-factor analysis, the NLR lost its independent prediction effect on pCR, the reason may be related to the high and low neutrophil count, the effect of NLR on pCR may mainly depend on the role of lymphocytes, there was no significant correlation between NLR and DFS before treatment, however, bias in the process of case collection cannot be ruled out. In addition, previous studies have suggested that high NLR and PLR may adversely affect the prognosis of breast cancer patients, in this study, in patients who received NAC but did not achieve pCR, patients with high NLR and high PLR had a worse prognosis, as the NLR and PLR values increase, it has certain prognostic value for breast cancer patients.
Most studies have shown that peripheral inflammatory markers are associated with breast cancer prognosis, however, there are few studies on peripheral inflammatory markers and the efficacy of neoadjuvant chemotherapy. Studies have shown that, a high level of NLR before treatment is significantly associated with poor prognosis in breast cancer patients (Wariss et al., 2017; Zhang et al., 2017). In the same way, a high level of PLR also indicates a poor prognosis of malignant tumors (Eruslanov et al., 2017), but the prognosis of a tumor reflects its ability to grow and invade, the efficacy of neoadjuvant chemotherapy can reflect the sensitivity of tumor to chemotherapy drugs. The correlation between inflammatory markers and the efficacy of neoadjuvant chemotherapy is influenced by various factors such as blood test results, different studies reported different NLR and PLR cutoff values, the prognostic value of peripheral blood NLR and PLR levels in breast cancer patients before treatment remains to be further studied, at the same time, whether monitoring changes in NLR and PLR levels during disease treatment and follow-up are conducive to early screening of recurrent lesions remains to be verified.
To sum up. high levels of NLR and PLR in peripheral blood before treatment predicted poorer DFS in breast cancer patients receiving NAC, it also has predictive value for patients whose NAC does not reach pCR. Elevated NLR and PLR levels suggest a worse prognosis, however, there are some differences in the cutoff values used by different studies, more prospective clinical trials are needed to further verify the correlation between peripheral inflammatory markers and the efficacy of neoadjuvant chemotherapy for breast cancer.
Authors’ contributions
Liu Shihao and Zhang Xin designed this study. Hu Guoqing collected data. Liu Shihao and Cui Ruina wrote this manuscript. Zhang Xin and Hu Guoqing revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study was supported by Handan Municipal Bureau of Science and Technology (No. 1823208078ZC), Name: Study on the Influence of TE-1 expression in neoadjuvant chemotherapy for breast cancer and its clinical significance. We would like to extend our sincere gratitude to our departmental chair for their support. Additionally, we would like to give many thanks to our physicians, engineers, and nurses as well as the other staff of the department.
Asano Y., Kashhiwagi S., Onoda N., et al., 2016, Predictive value of neutrophil/lymphocyte ratio for efficasy of preoperative chemo-therapy in Triple-Negative breast cacer, Ann Surg Oncol, 23(24): 1104-1110
https://doi.org/10.1245/s10434-015-4934-0
PMid:26511266 PMCid:PMC4773470
Azab B., Bhatt V.R., Phookan J. et al., 2012, Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients, Ann Surg Oncol, 19(1): 217-224
https://doi.org/10.1245/s10434-011-1814-0
PMid:21638095
Brar H.D., Anderson S., Smith R.E., et al., 2006, Sequential preoperative or poatoperative docetaxel added to peeoperative doxorubicin pluscyclophosphamide for operable breast cancer: National surgical adju-vant breast and bowel project protocol B-27, Clin Oncol, 24(13): 2019-2027
https://doi.org/10.1200/JCO.2005.04.1665
PMid:16606972
Chen M.S., Zhang Y.L., Hou L.M., et al., 2018, The relationship between the ratio of neutrophils to lymphocytes and the ratio of platelets to lymphocytes in peripheral blood and the prognosis of breast cancer patients with neoadjuvant chemotherapy, Chinese Journal of Mammary Diseases (Electronic Edition), 12(2): 93-99
Chen Y., Chen K., Xiao X.Y. et al., 2016, Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chm-otherapy as an independent prognostic indicator in breast cancer patients: a retrospective study, BMC Cancer, 16(1): 320-329
https://doi.org/10.1186/s12885-016-2352-8
PMid:27198767 PMCid:PMC4872336
Coffelt S.B., Wellenstein M.D., and de Visser K.E., 2016, Neutrophils in cancer: neutral no more[J]. Nat Rev Cancer, 16(7): 431-446
https://doi.org/10.1038/nrc.2016.52
PMid:27282249
Duan J., Pan L., and Yang M., 2018, Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: A meta-analysis, Medicine (Baltimore), 97(9): 133-139
https://doi.org/10.1097/MD.0000000000013340
PMid:30544398 PMCid:PMC6310509
Duffaud F., and Therasse P., 2000, New guidelines to evaluate the response to treatment in solid tumors, Bull Cancer, 87(12): 881-886
Eruslanov E.B., Bhojnagarwala P.S., Quatromoni J.G. et al., 2014, Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer, J Clin Invest, 124(12): 5466-5480
https://doi.org/10.1172/JCI77053
PMid:25384214 PMCid:PMC4348966
Feliciano E.M., Kroenke C.H., Meyerhardt J.A., et al., 2017, Association of systemic in flammation and sarcopenia with survival in nonmetastatic colorectal cancer results from the c scans study, Jama Oncol, 3(12): 172-178
https://doi.org/10.1001/jamaoncol.2017.2319
PMid:28796857 PMCid:PMC5824285
Gunduz S., Goksu S.S., Arslan D. et al., 2015, Factors affecting disease-freesurvival in patients with human epidermal growth factor receptor 2-positive breast cacer who receive adjuvant trastuzumab, Mol Clin Oncol, 3(5): 1109-1112
https://doi.org/10.3892/mco.2015.610
PMid:26623060 PMCid:PMC4534854
Liang W., and Ferrara N., 2016, The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis, Cancer Immunol Res, 4(2): 83-91
https://doi.org/10.1158/2326-6066.CIR-15-0313
PMid:26839309
Michaud D.S., Houseman E.A., Marsit C.J., et al., 2015, Understanding the role of the immune system in the development of cancer: new opportunities for population-baser research, Cancer Epidemiol Biomarkers Prev, 24(12): 1811-1819
https://doi.org/10.1158/1055-9965.EPI-15-0681
PMid:26396143
Ngui N.K., French J., Kilby C.J. et al., 2016, Axillary reverse mapping in patients with breast cancer: Is it oncologically safe, SurgOncol, 113(7): 726-731
https://doi.org/10.1002/jso.24231
PMid:27041002
Powell D.R., and Huttenlocher A., 2017, Neutrophils in the tumor mincroenvioment, Trends Lm munol, 37(1): 41-52
https://doi.org/10.1016/j.it.2015.11.008
PMid:26700397 PMCid:PMC4707100
Raungkawemanee S., Tangjitamol S., Manusirivithaya S., et al., 2012, Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer, J Gynecol Oncol, 23(4): 265-273
https://doi.org/10.3802/jgo.2012.23.4.265
PMid:23094130 PMCid:PMC3469862
Swierczak A., Mouchemore K.A., Hamilton J.A., et al., 2015, Neutrophils: important contributors to tumor progression and metastasis, Cancer Metastasis Rev, 34(4): 735-751
https://doi.org/10.1007/s10555-015-9594-9
PMid:26361774
Wariss B.R., de Souza Abrahão K., de Aguiar S.S. et al., 2017, Effec-tiveness of four inflammatory markers in predicting progno-sis in 237women with breast cancer, Maturitas, 4(15): 290-298
Zhang M., Huang X.Z., Song Y.X. et al., 2017, High Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopath-ological Characteristics in Patients with Breast Cancer: A Meta-Analysis, Biomed Res Int, 9(32): 50-59
https://doi.org/10.1155/2017/9503025
PMid:29082257 PMCid:PMC5610825
Zhao Y., Wang X.L., Zhang D.W., et al., 2019, Conversion of immunohistochemical markers and breast density are associated with pathological response and prognosis in very young breast cancer patients who fail to achieve a pathological complete response after neoadjuvant chemotherapy, Cancer Management and Research, 11(2): 5677-5690
https://doi.org/10.2147/CMAR.S198844
PMid:31417311 PMCid:PMC6592039
. PDF(242KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Shihao Liu
. Xin Zhang
. Guoqing Hu
. Ruina Cui
Related articles
. Breast cancer
. Peripheral inflammatory markers
. Neoadjuvant chemotherapy
. Pathological complete remission
. Disease-free survival
Tools
. Email to a friend
. Post a comment