 Author
Author  Correspondence author
Correspondence author
Cancer Genetics and Epigenetics, 2024, Vol. 12, No. 1 doi: 10.5376/cge.2024.12.0001
Received: 20 Nov., 2023 Accepted: 21 Dec., 2023 Published: 01 Jan., 2024
Wang T., 2024, The application prospects of immunomodulators in cancer treatment, Cancer Genetics and Epigenetics, 12(1): 1-7 (doi: 10.5376/cge.2024.12.0001)
Cancer is a significant global health issue with increasing incidence and mortality rates. Traditional cancer treatment methods, including surgical removal, radiotherapy, and chemotherapy, have achieved some efficacy to a certain extent but still pose many limitations and side effects. Therefore, finding new treatment strategies has become a focal point in current cancer research. Immune modulators, as a novel approach to cancer treatment, have the characteristics of regulating the immune system and enhancing the body's immune response, showing promising results in clinical practice. However, there is currently some controversy and uncertainty regarding the prospects of immune modulators in cancer treatment. Thus, this review aims to summarize the application prospects of immune modulators in cancer treatment, explore their mechanisms of action, clinical application status, and development trends, providing a reference for further research and clinical practice.
Cancer causes millions of deaths annually, and despite the partial success of traditional cancer treatments such as surgery, radiotherapy, and chemotherapy, they have limitations and side effects. These include damage to normal cells, the development of drug resistance, and the recurrence of tumors post-treatment. Therefore, finding new treatment strategies has become a key focus in current cancer research. Immune modulators have emerged as a crucial area of study in the field of cancer treatment. The immune system plays a critical role in combating cancer by recognizing and eliminating abnormal cells, thus preventing the development and spread of tumors (Omar et al., 2019). However, cancer cells often evade attacks from the immune system through various mechanisms, leading to immune tolerance and escape. Therefore, by modulating the immune system and enhancing the body's immune response, it is possible to effectively suppress the growth and metastasis of tumors.
The mechanisms of action of immune modulators are diverse, including the activation of immune cells, enhancement of anti-tumor immune responses, and inhibition of tumor immune escape. The application of these immune modulators provides new perspectives and approaches for cancer treatment. Currently, immune modulators have shown encouraging results in clinical applications. Immune checkpoint inhibitors have been widely used in the treatment of various cancers, such as melanoma, non-small cell lung cancer, and renal cell carcinoma (Shiravand et al., 2022), achieving significant therapeutic effects. Additionally, other types of immune modulators, such as cytokines and tumor vaccines, have demonstrated certain potential in clinical trials.
However, immune modulators still face challenges and limitations in cancer treatment. Variability in immune responses among different tumor types and individuals necessitates further research and optimization of the selection and application of immune modulators. The side effects and safety of immune modulators also require careful consideration, especially in long-term and combination therapies. Additionally, the efficacy and resistance issues of immune modulators need further investigation and resolution.
This review provides an overview of immune modulators, with a focus on their application in cancer treatment, including their anti-tumor mechanisms and application cases in different types of cancer. Simultaneously, it discusses the development trends and prospects of immune modulators, encompassing the development of novel immune modulators, the advancement of personalized immune therapies, and the combined application of immune modulators with other treatment methods. Finally, the review summarizes the prospects of immune modulators in cancer treatment, offering insights into future research directions. It is hoped that this review provides a comprehensive understanding of the application prospects of immune modulators in cancer treatment, offering a scientific basis for clinical practice, providing more effective and safe treatment strategies for cancer patients, and contributing to further advancements in the field of cancer research.
1 Overview of Immune Modulators
1.1 Definition and classification of immune modulators
GImmune modulators refer to drugs or therapeutic methods capable of regulating the function of the immune system. Different types of immune modulators may vary in treatment mechanisms, indications, and side effects. Therefore, their selection and application should be based on specific circumstances. Immune checkpoint inhibitors are immune checkpoints that activate immune cells to attack tumors by blocking inhibitory signals between tumor cells and immune cells. Common immune checkpoints include anti-CTLA-4 antibodies (such as ipilimumab), anti-PD-1 antibodies (such as pembrolizumab), and anti-PD-L1 antibodies (such as atezolizumab).
Cytokines are a class of protein molecules that can enhance the activity of immune cells and promote immune responses (Liu et al., 2022). Common cytokines include interferons (such as interferon-α and interferon-γ), interleukins (such as interleukin-2 and interleukin-12), among others. Tumor vaccines represent a kind of vaccine capable of eliciting a specific immune response in the body, recognizing and eliminating tumor cells. Tumor vaccines may include tumor-related antigens, tumor cells, or their products, among other components. Apart from the types of immune modulators mentioned above, there are also other types such as immune cell therapy (e.g., CAR-T cell therapy), and immune adjuvants (e.g., liposomes and adjuvants), etc.
1.2 Mechanisms of action of immune modulators
Different types of immune modulators may have distinct mechanisms of action, and these mechanisms can be influenced by various factors such as dosage, administration route, and the target of treatment. Therefore, when using immune modulators, it is necessary to make selections and applications based on specific circumstances. Certain immune modulators can inhibit the activity of the immune system, reduce the function and quantity of immune cells, thereby diminishing the intensity of immune responses. This mechanism is commonly employed in the treatment of autoimmune diseases. Other immune modulators can enhance the activity of the immune system, promote the function and quantity of immune cells, thereby intensifying the effectiveness of immune responses. This mechanism is often used in the treatment of infectious diseases and tumors, such as interferons and interleukins.
Certain immune modulators can regulate the balance of the immune system, maintaining appropriate control during immune responses. This mechanism is often employed in the treatment of diseases associated with immune dysregulation, such as immune checkpoint inhibitors (e.g., anti-CTLA-4 antibodies and anti-PD-1 antibodies) (Seidel et al., 2018). Some immune modulators can enhance the immune system's memory and recognition capabilities for specific antigens, thereby strengthening the persistence and specificity of immune responses. This mechanism is commonly used in the fields of vaccines and immune cell therapy.
1.3 The current clinical application of immunomodulators
Immunomodulators are widely utilized in the treatment of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and others. Commonly used immunomodulators include corticosteroids, immunosuppressants (such as cyclosporine A, methotrexate), and others. Immunomodulators are employed post-organ transplantation to suppress the host immune system's rejection response toward the transplanted organ. Commonly used immunosuppressants include cyclosporine A, tacrolimus, and others.
Immunomodulators play a crucial role in the treatment of hematological malignancies such as leukemia and lymphoma. For instance, immunostimulants like interferons and interleukins can be employed to enhance the immune system's ability to attack tumors. Immunomodulators are also utilized in the treatment of diseases related to immune dysregulation, such as immunodeficiency disorders and allergic conditions. Immune checkpoint inhibitors (such as anti CTLA-4 antibodies and anti PD-1 antibodies) are a commonly used class of immune modulators. Furthermore, immunomodulators are used to boost the effectiveness of vaccines, thereby enhancing vaccine protection. For example, adjuvants (such as aluminum salts) can augment the immunogenicity of vaccines.
2 Immunomodulator Application in Cancer Treatment
2.1 Mechanisms of immunomodulators in antitumor therapy
The mechanisms through which immunomodulators act in anticancer treatment primarily involve enhancing immune responses, suppressing immune inhibition, and modulating immune balance. Immunomodulators can augment the body's immune system's ability to recognize and attack tumor cells. For instance, immunostimulants like interferons and interleukins can activate immune cells, increase the production and activation of tumor-specific T cells, and promote immune cell-mediated cytotoxicity against tumor cells.
There are some immunosuppressive factors in the tumor microenvironment, such as the overexpression of immune checkpoint molecules (e.g., PD-1, CTLA-4), which inhibit the activation and cytotoxicity of immune cells against tumor cells (Huang et al., 2020). Immunomodulators can counteract the effects of immunosuppressive factors by inhibiting their actions, relieving the inhibition of immune cells, and enhancing the immune cells' ability to attack tumor cells. For example, anti-PD-1 antibodies and anti-CTLA-4 antibodies can block the PD-1 and CTLA-4 signaling pathways, restoring the activation and cytotoxic functions of immune cells.
During the process of tumor development, the immune system often exists in a state of immune balance, wherein tumor cells evade immune system attacks by modulating the functions of immune cells. Immunomodulators can adjust this immune balance, enabling the immune system to regain its ability to target tumor cells. For example, immunosuppressants can inhibit the inhibitory functions of immune cells, enhancing the cytotoxic effects of immune cells against tumor cells.
It should be noted that different types of tumors and individual immune states may have an impact on the anti-tumor effect of immune modulators. Therefore, when employing immunomodulators for anticancer treatment, personalized treatment plans should be formulated based on individual circumstances, and close monitoring of the patient's immune status and drug-related side effects is essential.
2.2 Application cases of immunomodulators in the treatment of different types of cancer
The application of immunomodulators may vary across different types of cancer, and specific treatment plans need to be determined based on the patient's condition and immune status. Additionally, the use of immunomodulators may give rise to certain side effects, such as immune-related toxic reactions. Therefore, close monitoring of the patient's immune status and drug-related side effects is crucial throughout the course of treatment.
PD-1 antibodies (such as Pembrolizumab and Nivolumab) have been widely employed in the treatment of melanoma (Huang et al., 2021). Melanoma patients often exhibit demonstrate of PD-1, and anti-PD-1 antibodies can block the binding of PD-1 to its ligand PD-L1, restoring T cell cytotoxicity against tumor cells, thereby enhancing therapeutic efficacy. CTLA-4 antibody (such as Ipilimumab) is used in the treatment of metastatic melanoma (Figure 1). CTLA-4 is an immune inhibitory molecule, and anti-CTLA-4 antibodies can block CTLA-4's function, enhancing T cell activation and cytotoxicity, thereby inhibiting the growth and spread of melanoma.
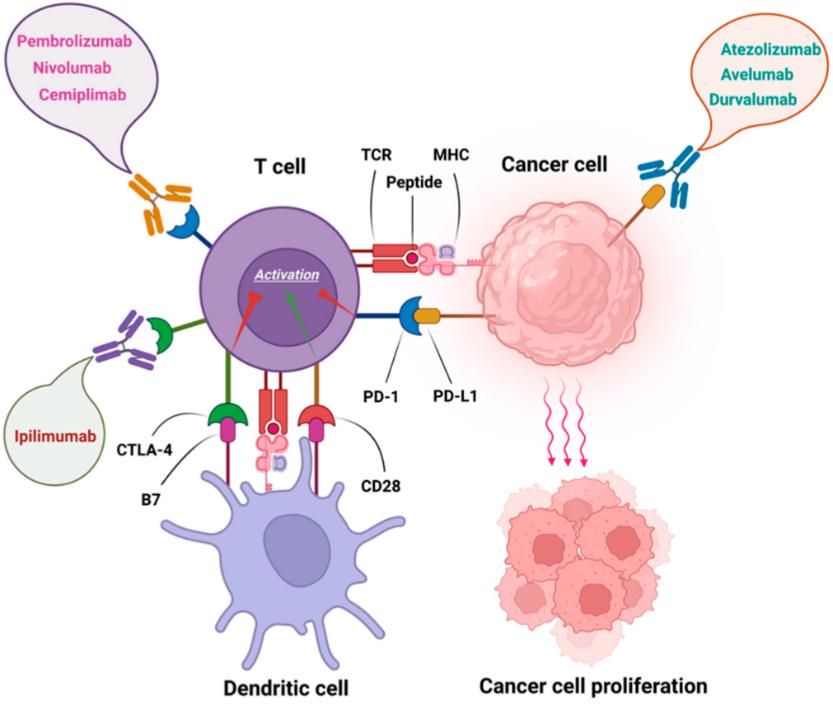 Figure 1 FDA approved immune checkpoint inhibitors (Shiravand et al., 2022) Note: Pembrolizumab, Nivolumab, and Cemiplimab as anti PD-1 antibodies; Ipilimumab as anti CTLA-4 antibody and Atezolizumab, Avelumab, and Durvalumab as anti PD-L1 antibodies |
Interferon is an immunostimulant widely utilized in the treatment of renal cell carcinoma. Interferon enhances the immune cell's ability to attack tumor cells and inhibits the growth and spread of tumor cells. Interleukin-2 (IL-2) is an immunostimulant used in the treatment of melanoma and renal cell carcinoma. IL-2 activates immune cells, increases the production and activation of tumor-specific T cells, and promotes the immune cell-mediated cytotoxicity against tumor cells.
2.3 Advantages and challenges of immunomodulators in cancer treatment
Immunomodulators in cancer treatment offer advantages including targeting the immune system, providing durable therapeutic effects, and being applicable to various cancer types. Immunomodulators can directly influence the immune system, activating or inhibiting specific immune cells or molecules, thereby enhancing the immune system's ability to attack tumors. Compared to traditional chemotherapy and radiation therapy, immunomodulators can generate sustained therapeutic effects. Once the immune system's ability to target tumors is activated, immune cells can continually eliminate tumor cells, reducing the risk of recurrence and metastasis. Immunomodulators find applications in the treatment of various cancer types, including melanoma, lung cancer, breast cancer, colorectal cancer, among others. This makes immunomodulators a widely applicable and versatile treatment approach.
One of the challenges in cancer treatment with immunomodulators is the development of resistance, where some patients may exhibit resistance to immunomodulator therapy (Da Silva et al., 2019). Tumor cells can evade immune cell attacks by altering immune escape mechanisms, thereby diminishing the effectiveness of immunomodulators. Additionally, the use of immunomodulators may trigger immune-related toxic reactions, such as autoimmune diseases caused by immune cell attacks on normal tissues. These toxic reactions can negatively impact the quality of life and treatment outcomes for patients.
Furthermore, individual variations in patient responses to immunomodulators may exist. Some patients may respond well to immunomodulator therapy, while others may not. The high cost of immunomodulator treatment may limit access for some patients. Additionally, some immunomodulators have not been approved by insurance companies, making it difficult for patients to obtain these treatments.
3 Trends in the Development of Immunomodulators
3.1 Development of novel immunomodulators
The development of novel immunomodulators represents a crucial research direction in the field of cancer treatment. Scientists are continually striving to identify new immunomodulators to enhance the effectiveness of cancer therapy and reduce side effects. These research endeavors hold the promise of bringing about breakthroughs in cancer treatment. However, it is important to note that the development of novel immunomodulators is a complex and lengthy process, necessitating rigorous laboratory research and clinical trials before they can be ultimately applied in clinical settings.
CAR-T cell therapy is a treatment method that utilizes engineered T cells to target and attack cancer cells (Jiang et al., 2022). Scientists are actively researching ways to enhance the design of CAR-T cells to improve their therapeutic efficacy and safety. While immune checkpoint inhibitors have achieved significant success, a portion of patients still does not respond effectively to this treatment. Consequently, researchers are investigating the combination of immune checkpoint inhibitors with other treatment modalities such as chemotherapy, radiation therapy, and targeted therapy to enhance overall treatment outcomes.
Scientists are also on the lookout for novel immunomodulators to activate the immune system's ability to target tumors. This includes the search for new immune checkpoint molecules, immune cell activators, tumor-associated antigens, and more. The emergence of gene editing technologies (such as CRISPR-Cas9) provides a new tool for the development of immunomodulators. Researchers can use gene editing techniques to modify the functions of immune cells, enabling them to better target and attack cancer cells. The microbiome plays a crucial role in immune system regulation. Scientists are exploring how to enhance the immune system's ability to target tumors by modulating the gut microbiome.
3.2 Development of personalized immunotherapy
Personalized immunotherapy is an approach to individualized treatment based on patient-specific differences. It involves designing precise treatment plans considering factors such as the patient's genetic background, immune status, and other variables, aiming for optimal therapeutic outcomes. Personalized immunotherapy represents a significant trend in the future of cancer treatment. Although it currently faces numerous challenges, continuous technological advancements are expected to bring about more precise and effective treatment strategies in clinical practice.
With the continuous advancement of molecular biology and genomics technologies, scientists can acquire patients' molecular characteristics through methods such as gene testing and genetic sequencing. Based on this information, individualized treatments like targeted therapy can be implemented. While immunotherapeutic drugs show promising prospects in cancer treatment, there are still cases where the therapeutic outcomes are suboptimal. With in-depth research on the mechanisms of cancer immunotherapy, scientists can develop more precise treatment plans from the perspective of individual patient differences, such as personalized application of immune checkpoint inhibitors.
Cellular immunotherapy involves utilizing biotechnology to transform a patient's own immune cells into cells with anti-tumor capabilities, which are then reintroduced into the patient's body for treatment (Huang et al., 2019). Personalized cellular immunotherapy can be precisely designed based on factors such as the patient's immune status and the characteristics of immune cells, aiming to achieve better treatment outcomes.
3.3 Combination application of immunomodulators with other treatment methods
The combined application of immunomodulators with other treatment methods represents a comprehensive therapeutic strategy aimed at enhancing treatment outcomes through the synergistic effects of different therapeutic approaches. The combined use of immunomodulators with other treatment methods requires the individualized design of treatment plans based on the specific conditions of each patient, along with vigilant monitoring and assessment in clinical practice. Additionally, the combination approach may increase treatment side effects and risks, necessitating guidance under the supervision of a qualified medical professional.
Chemotherapy is a treatment method that utilizes chemical drugs to kill cancer cells. However, chemotherapy can also cause damage to normal cells and the immune system. Immunomodulators can enhance immune system function, improve the body's tolerance to chemotherapy, and enhance the effectiveness of chemotherapy. Targeted therapy is a treatment method that inhibits the growth and spread of cancer cells by targeting specific molecular targets on cancer cells. Immunotherapy activates the immune system, enhancing its ability to attack cancer cells. The combination of these approaches can improve treatment outcomes and reduce the occurrence of drug resistance (Barbari et al., 2021).
Radiation therapy is a treatment method that uses high-energy radiation to kill cancer cells (Figure 2). However, radiation therapy can also cause damage to normal cells and the immune system. Immunomodulators can enhance immune system function, improve the body's tolerance to radiation therapy, and enhance the effectiveness of radiation therapy. Surgery is a treatment method that involves the removal of tumor tissue. Immunotherapy can activate the immune system, eliminating residual cancer cells and reducing the risk of recurrence and metastasis. The combination of these approaches can improve the effectiveness of surgery and reduce the likelihood of postoperative recurrence.
 Figure 2 Cystic fibrosis |
3.4 Potential applications in cancer prevention and early diagnosis
Immunomodulators have potential applications in cancer prevention and early diagnosis. By modulating the function of the immune system, immunomodulators can enhance the body's ability to clear precancerous lesions, thereby preventing their progression into cancer. For example, certain immunomodulators can augment immune cell recognition and cytotoxicity against abnormal cells, reducing the development of precancerous lesions.
Immunomodulators can reduce the risk of cancer by enhancing the functionality of the immune system, thereby improving the body's ability to eliminate cancer cells. For instance, certain immunomodulators can boost immune cell attack on cancer cells, reducing their survival and spread. By modulating the immune system's function, immunomodulators can enhance the body's ability to recognize cancer cells, thereby helping to detect cancer early. For example, some immunomodulators can enhance immune cell recognition of cancer cell-specific antigens, thereby increasing the detection rate of early-stage cancer.
4 Summary and Outlook
Cancer poses a significant global health challenge, and traditional treatment methods such as chemotherapy and radiation therapy have certain limitations. Immunotherapy, as an emerging treatment strategy, has made significant strides by harnessing the patient's own immune system to target cancer cells. Immune checkpoint inhibitors, among the most successful immunotherapeutic approaches, activate the patient's immune system to attack cancer cells by inhibiting the action of immune checkpoint molecules. These drugs have demonstrated notable therapeutic effects in various cancer types, including melanoma and non-small cell lung cancer. However, immunotherapy still faces challenges such as immunoresistance and side effects.
Immunotherapy can lead to immune resistance in some patients. Future research will explore the mechanisms underlying immune resistance and seek methods to overcome it, such as developing new immunomodulators, implementing combination therapy strategies, and enhancing immune cell functionality. The application of immunotherapy needs to consider individual differences and tumor characteristics. Future research will focus on developing personalized immunotherapy strategies, including predictive models based on tumor genomics and immunohistology, as well as tailored treatment plans for individual patients.
The potential application of immunomodulators in early cancer diagnosis and prevention holds promise (Zhu et al., 2023). Subsequent research will investigate the mechanisms of action of immunomodulators in early cancer diagnosis and prevention, along with the development of corresponding treatment strategies. The application of immunomodulators requires consideration of their safety and side effects. Future research will continue to focus on the safety of immune modulators and seek methods to reduce side effects, enhancing the acceptability and effectiveness of treatments.
In conclusion, the outlook for the application of immunomodulators in cancer treatment is very promising, but further research and validation are needed. With the continuous progress of scientific and technological advancements, we anticipate the emergence of more innovative immunomodulators and treatment strategies, bringing improved therapeutic outcomes and survival rates for cancer patients.
Acknowledgments
From the selection of the topic for this study to the final completion of the project, gratitude is extended to Ms. Yeping Han for her valuable input and suggestions.
Barbari C., Fontaine T., Parajuli P., Lamichhane N., Jakubski S., Lamichhane P., and Deshmukh R.R., 2020, Immunotherapies and combination strategies for immuno-oncology, Int. J. Mol. Sci., 21: 5009.
https://doi.org/10.3390/ijms21145009
PMid:32679922 PMCid:PMC7404041
Da Silva C.G., Camps M.G.M., Li T.M.W.Y., Chan A.B., Ossendorp F., and Cruz L.J., 2019, Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines, Biomaterials, 220: 119417.
https://doi.org/10.1016/j.biomaterials.2019.119417
PMid:31419588
Huang D., Gong C., and Song E.W., 2019, Adoptive immune cell therapy for malignant tumors moves from “individualization” to “precision”, Shengming Kexue (Chinese Bulletin of Life Sciences), 31(7): 651-659.
Huang Q., Zheng Y., Gao Z., Yuan L., Sun Y., and Chen H., 2021, Comparative efficacy and safety of PD-1/PD-L1 inhibitors for patients with solid tumors: a systematic review and bayesian network meta-analysis, J. Cancer, 12: 1133.
https://doi.org/10.7150/jca.49325
PMid:33442411 PMCid:PMC7797652
Huang S.J., Qiu X.D., Li W.Y., Tang K.R., Wu Q., Deng H.Y., Deng L.F., and Huang L., 2020, Cellular crosstalk and tumorigenic mechanisms in tumor microenvironment, Shengming Kexue (Chinese Bulletin of Life Sciences), 32(4): 315-324.
Jiang Y., Wen W.H., Yang F., Nie D., Zhang W.H., and Qin W.J., 2022, Research progress of multi-target CAR-T cell therapy for cancer, Zhongliu Fangzhi Yanjiu (Cancer Research on Prevention and Treatment), 49(7): 709-714.
Liu L., Jiao P.T., Wang M., Li J., Sun L., Fan W.H., and Liu W.J., Effects of chicken interferon-γ and interleukin-2 on cytokines related to Th1 cell differentiation in peripheral blood, Shengming Gongcheng Xuebao (Chinese Journal of Biotechnology), 38(9): 3329-3343.
Omar H.A., El-Serafi A.T., Hersi F., Arafa E.S.A., Zaher D.M., Madkour M., Arab H.H., and Tolba M.F., 2019, Immunomodulatory MicroRNAs in cancer: targeting immune checkpoints and the tumor microenvironment, The FEBS Journal, 286(18): 3540-3557.
https://doi.org/10.1111/febs.15000
PMid:31306553
Seidel J., Otsuka A., and Kabashima K., 2018, Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations, Front. Oncol., 8: 86.
https://doi.org/10.3389/fonc.2018.00086
PMid:29644214 PMCid:PMC5883082
Shiravand Y., Khodadadi F., Kashani S.M.A., Hosseini-Fard S.R., Hosseini S., Sadeghirad H., Ladwa R., Byrne K.O., and Kulasinghe A., 2022, Immune checkpoint inhibitors in cancer therapy, Current Oncology, 29(5): 3044-3060.
https://doi.org/10.3390/curroncol29050247
PMid:35621637 PMCid:PMC9139602
Zhu X.X., Chen H.Z., Cui K.Y., Zhong W., Peng X.S., Zhou Y.B., Current situation and prospect of exosomes application in tumor diagnosis and treatment, Zhongguo Yixue Daobao (China Medical Herald), 20(9): 41-45.
. PDF(284KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Tina Wang
Related articles
. Immune modulators
. Cancer treatment
. Immune system
. Body immune response
. Development trends
Tools
. Email to a friend
. Post a comment