 Author
Author  Correspondence author
Correspondence author
Cancer Genetics and Epigenetics, 2024, Vol. 12, No. 2 doi: 10.5376/cge.2024.12.0009
Received: 25 Jan., 2024 Accepted: 28 Feb., 2024 Published: 11 Mar., 2024
Zhou J.Y., 2024, The Application of genomics in personalized cancer therapy, Cancer Genetics and Epigenetics, 12(1): 66-74 (doi: 10.5376/cge.2024.12.0009)
Genomics provides precise tools and methods for personalized cancer treatment by revealing the genomic characteristics of cancer. Through the practical application of typical cases, it demonstrates its great potential in improving treatment efficacy and patient survival rate, indicating that genomics will play a more important role in cancer treatment in the future. This review summarizes the genomic characteristics of cancer, including its variations, mutations, and roles in the occurrence, development, and metastasis of cancer, emphasizing the guiding significance of genomics for cancer treatment. It elaborates on the basic principles of personalized cancer treatment, and further analyzes the practical application effect and patient survival rate of genomics in personalized cancer treatment through specific cases. It discusses the development trend of genomics treatment technologies and methods, as well as its application prospects in personalized cancer treatment. This manuscript aims to reveal its key role in improving treatment efficacy and patient survival rate, and explore future development directions, with a view to providing new treatment strategies and methods for the field of cancer treatment.
Cancer, a disease that often strikes fear into the hearts of many, has long been a formidable challenge in the medical field. In recent years, with the rapid advancements in genomics, our understanding of cancer has moved from a superficial level to a molecular and genetic one, paving the way for personalized treatment options.
The significance of genomics in cancer treatment cannot be overstated. Each individual's genome is unique, and cancer is driven by specific mutations within these genomes. By thoroughly studying these mutations, we can better understand the mechanisms of cancer initiation, progression, and metastasis, thereby tailoring the most effective treatment plans for each patient (Besser et al., 2018). Personalized medicine, a direct application of genomics in cancer therapy, is fundamentally changing the paradigm of cancer treatment. The traditional "one-size-fits-all" treatment approach is gradually being replaced by precision therapies based on patient-specific genomic information (Mook et al., 2018).
Genomics provides essential tools for personalized cancer treatment. The development of high-throughput sequencing technologies allows for the rapid acquisition of a patient's complete genomic profile, while powerful bioinformatics tools help interpret this data and identify gene mutations closely linked to cancer development. Based on this information, the most appropriate targeted drugs or immunotherapy options can be selected for patients, maximizing the effectiveness of the treatment (Gwinn et al., 2019).
This paper aims to provide a comprehensive overview of the current status and trends in the application of genomics in personalized cancer treatment. It discusses the genomic characteristics of cancer and the basic principles and implementation methods of personalized cancer therapy. Through specific case studies, it examines the practical effectiveness of genomics in personalized treatment and explores the future directions of genomic therapy techniques and methods. It is hoped that this paper will serve as a valuable reference for researchers and clinicians in the field of cancer treatment, promoting the broader application of genomics in personalized cancer therapy.
1 Genomic Characteristics of Cancer
1.1 Genomic variations and mutations in cancer
Genomic variations and mutations are crucial in the initiation, progression, and metastasis of cancer. These changes are not only alterations in single genes but involve multiple genes, signaling pathways, and complex network interactions. They can occur in the coding regions, regulatory areas, or non-coding regions of genes, leading to altered protein functions, abnormal gene expression, and uncontrolled cell growth and division (Preethi et al., 2021).
Common types of genomic variations in cancer include point mutations, insertions, and deletions, which can result in the loss or gain of protein functions. Additionally, changes in chromosomal structures such as translocations, inversions, and deletions can affect the expression and regulation of multiple genes. The accumulation and interaction of these mutations and variations in cancer cells confer malignant phenotypes such as limitless proliferation, evasion of immune surveillance, and resistance to apoptosis.
1.2 Role of genomics in cancer initiation, progression, and metastasis
Cancer often begins with minor genomic variations that may arise from environmental factors, genetic predispositions, or random errors. Over time, these variations accumulate and expand, leading to uncontrolled cell growth and division, ultimately forming visible tumors. Genomics plays a critical role in the initiation, development, and spread of cancer.
During cancer progression, genomics reveals how cancer cells evade the immune system, resist apoptosis, and acquire enhanced growth and invasive capabilities. In-depth genomic studies have identified mutations in various cancer-related genes, such as the inactivation of tumor suppressor genes and activation of oncogenes, playing key roles in cancer advancement (Yuan et al., 2019).
Cancer metastasis, the spread of cancer cells from the primary site to other parts of the body, involves multiple complex steps. Genomics has shown how cancer cells modify their adhesiveness, motility, and invasiveness to facilitate this process. Additionally, it reveals how cancer cells interact with the host environment to exploit host cells for nutrients and support, and how they evade host immune attacks.
1.3 Implications of genomic research for cancer treatment
In-depth study of the genomic characteristics of cancer allows for a more accurate understanding of the nature and mechanisms of cancer, providing more precise and personalized guidance for treatment strategies.
Genomic research has identified key gene mutations and signaling pathway abnormalities in cancer, offering direct targets for targeted therapy and immunotherapy. For instance, specific gene mutations can be targeted with drugs that precisely attack cancer cells while minimizing toxicity to normal cells. Similarly, genomic studies have uncovered molecular mechanisms related to cancer immune evasion, leading to the development of immunotherapies that activate the patient's immune system to attack cancer cells (Aiello et al., 2018).
Furthermore, genomic research can predict cancer prognosis and the risk of recurrence, providing more personalized treatment and management plans for patients. Comprehensive analysis of the cancer genome enables assessment of tumor type, stage, molecular characteristics, and more, predicting responses and efficacy to different treatment regimens. This helps doctors select the most suitable treatment options for patients and adjust strategies in time to improve treatment outcomes.
2 Principles of Personalized Cancer Treatment
2.1 Concept and principles of personalized medicine
Personalized medicine, also known as precision medicine or tailored medicine, is an emerging medical model that emphasizes creating and optimizing treatment plans based on the unique physiological, genetic, and environmental characteristics of each patient. The concept of personalized medicine is founded on a profound understanding of the human genome and biodiversity, challenging the traditional “one-size-fits-all” treatment approach in conventional medical practice. It proposes more refined and individualized methods for disease prevention, diagnosis, and treatment (Guthrie et al., 2019).
The principles of personalized medicine primarily rely on advancements in two key areas: genomics and bioinformatics. Genomics provides the techniques and methods to study human genes, gene variations, and gene expressions, while bioinformatics is responsible for analyzing these extensive genomic data to extract crucial information related to disease onset and progression. By integrating knowledge and technology from these domains, physicians can perform in-depth analyses of a patient’s genome, identify gene mutations related to diseases, predict disease risks and progression, and tailor the most appropriate treatment plans for patients.
Personalized medicine not only improves treatment outcomes and reduces unnecessary medical expenses but also enhances patients' quality of life. By precisely selecting drugs and treatment methods, ineffective treatments and the side effects and complications of overtreatment can be avoided. Furthermore, personalized medicine encourages patients to actively participate in their medical decision-making process, enhancing communication and trust between doctors and patients.
2.2 Utilizing genomic technologies for personalized cancer treatment
Utilizing genomic technologies for personalized cancer treatment represents a revolutionary medical model that formulates customized treatment plans based on the unique genetic backgrounds and disease characteristics of patients. In personalized cancer treatment, genomic technologies play a crucial role (Hill et al., 2018).
High-throughput sequencing and other genomic technologies enable researchers to obtain comprehensive genomic information from patients, including gene sequences, expression levels, and variations. This data provides physicians with a rich source of information, allowing them to accurately understand the type and progression of a patient’s disease and thereby choose the most suitable treatment plans. Based on genomic data, physicians can predict patients' responses to specific drugs and their therapeutic effects, thus avoiding ineffective treatments and their associated side effects and complications. For instance, certain cancer patients may have specific gene mutations that cause resistance to some drugs. Through genomic testing, doctors can quickly identify these mutations and select alternative, more effective medications (Yamaguchi et al., 2018).
Moreover, genomic technologies also help researchers discover new therapeutic targets and develop novel drugs. By thoroughly studying cancer genomes, scientists can identify key genes and signaling pathways related to cancer development and progression, thereby developing targeted drugs or immunotherapies aimed at these targets. These new treatments offer higher selectivity and efficacy, providing cancer patients with better survival chances and quality of life.
2.3 Processes and methods of personalized treatment
2.3.1 Genomic Sequencing and Analysis
Genomic sequencing and analysis are the core components of the field of genomics, providing deep insights into an organism’s genome. Genomic sequencing involves using high-throughput sequencing technology to sequence the entire genome of an organism, thereby obtaining complete genomic information. During this process, researchers use advanced sequencing equipment and reagents to convert the genetic material of an organism into digital signals, thus obtaining genomic sequence data (Satta et al., 2018).
After sequencing, the next step is genomic analysis, which includes quality control of sequencing data, alignment to reference genomes, identification of gene variants, and analysis of gene expression, among other steps. By employing bioinformatics tools and algorithms, researchers can deeply mine genomic data to reveal the genomic structure, gene functions, regulatory mechanisms, and key mutations related to disease onset and progression (Figure 1).
 Figure 1 Typical Pathogen Genomics Workflow (Gregory et al., 2019) |
2.3.2 Selection of molecular targeted drugs
The selection of molecular targeted drugs is a significant advancement in the field of cancer treatment, based on genomic research findings. This approach precisely targets specific molecular abnormalities in cancer. Initially, it involves determining the genomic characteristics of a cancer patient, typically through sequencing and expression analysis of tumor samples. Through these analyses, researchers can identify gene mutations and abnormal signaling pathways closely related to cancer development and progression.
Based on the patient's genomic characteristics, physicians select appropriate molecular targeted drugs. These drugs typically target specific proteins or signaling pathways, inhibiting or activating these molecules to block cancer growth and spread. For example, some targeted drugs may focus on specific receptors on cancer cell surfaces, preventing their interaction with ligands and thus inhibiting cancer cell growth; others may target intracellular signaling pathways, blocking cancer cell proliferation and survival.
When selecting molecular targeted drugs, considerations also include the patient’s overall health, previous treatment responses, and the side effects of the drugs. As each patient's genomic characteristics are unique, the selection of molecular targeted drugs must be individualized. Physicians need to weigh the pros and cons according to the specific circumstances of the patient, devising the most suitable treatment plan.
2.3.3 Application of immunotherapy and immune checkpoint inhibitors
The application of immunotherapy and immune checkpoint inhibitors has brought revolutionary breakthroughs in cancer treatment. Immunotherapy leverages the human body’s own immune system, activating or restoring the function of immune cells to attack cancer cells. This method maximizes the use of the body’s natural defenses, making treatment more precise and with relatively fewer side effects.
Immune checkpoint inhibitors are a crucial class of drugs in immunotherapy, capable of suppressing immune checkpoint molecules on cancer cells, thereby releasing the inhibition of immune cells against cancer cells. This reactivates immune cells to recognize and attack cancer cells, restoring anti-tumor immune responses. Immune checkpoint inhibitors, such as PD-1 inhibitors and CTLA-4 inhibitors, have achieved significant clinical success in treating various cancers, significantly extending patients' survival periods and improving their quality of life.
3 The Application of Genomics in Personalized Cancer Treatment
3.1 Typical cases of genomics in personalized cancer treatment
CRISPR genome editing can be used for personalized cancer treatment. A study published by "Nature" magazine on November 10, 2022, reported significant advances in modified cells and their clinical trials in humans. This research, conducted by researchers at the University of California and the cell therapy company PACT Pharma, developed a method using the CRISPR-Cas9 genome editing system to insert cancer-specific T-cell receptors into the T cells of cancer patients, thereby generating personalized anti-cancer immune cells.
Leveraging the power of the human immune system to treat cancer is an attractive goal. T-cell receptors on the surface (a key part of the immune system involved in recognizing specific antigens and responding) can detect cancer cells because single mutations in the cancer cell genome alter surface proteins (MacCannell, 2019). Isolating these T-cell receptors that can detect cancer cells and using them to generate therapeutic T cells might pave a new way for treating intractable cancers (Figure 2).
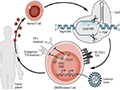 Figure 2 Gene-edited T cells for the treatment of cancer (Photo source: https://www.sohu.com/a/606549401_120554400) |
3.2 Analysis of treatment effects and patient survival rates in cases
In a Phase I clinical trial, the University of California and the cell therapy company PACT Pharma treated 16 patients with metastatic solid tumors (mostly colorectal cancer) who were unresponsive to standard therapies using genetically engineered T cells. These T cells expressed personalized T-cell receptors targeting individual cancer mutations. Among the 16 participants, the therapy stabilized the disease in 5 patients, while the condition of the other 11 patients progressed. Only 2 patients experienced adverse reactions due to T-cell therapy, whereas all patients suffered expected adverse reactions related to concurrent chemotherapy.
Although this method has limitations, such as characterizing potential antigens and the time required to isolate, clone, and test T-cell receptors, and the variable affinity of patient-specific T-cell receptors to their corresponding antigens, the clinical response benefits were limited. However, some processes were optimized during the trial, and there is room for further improvements. This research also demonstrated the potential viability of this treatment strategy.
3.3 Application and challenges of genomics in various types of cancer
Genomics is widely and deeply applied in various types of cancer, but it also faces many challenges. In terms of application, genomics provides a crucial foundation for precision medicine by deeply analyzing gene mutations in cancer cells. For instance, in lung cancer, genomics helps doctors identify mutations in key genes such as EGFR and KRAS, guiding the selection of targeted drugs. In breast cancer, the amplification status of the HER2 gene becomes an important indicator for assessing whether a patient is suitable for specific treatments. In gastrointestinal cancer, in-depth research on the genome has revealed multiple gene mutations related to tumor occurrence and development, providing clues for developing new treatment strategies.
However, genomics also faces challenges in cancer applications. Different types of cancers have distinct genomic characteristics, making the interpretation and analysis of genomic data complex. Even within the same type of cancer, genomic variations among different patients are significant, requiring doctors to have a high level of expertise and skills in formulating personalized treatment plans. The acquisition and analysis of genomic data require expensive equipment and skilled personnel, which somewhat limits its application in primary healthcare facilities. Additionally, the privacy protection of genomic data is an urgent issue to address; ensuring that patients' genetic information is not misused or leaked poses significant ethical and legal challenges.
Genomics still faces challenges in drug development and clinical trials. Although some drugs based on genomic discoveries have entered clinical use, many potential therapeutic targets still require further validation and research. Moreover, effectively integrating genomic data with clinical trial results to guide clinical practice remains a critical task.
4 Trends in Genomics Technologies and Methods for Treatment
4.1 Trends and future directions in genomics technologies
Genomics, as a core area of modern biology, is undergoing rapid development with limitless potential for the future. With continuous innovations in sequencing technologies, third-generation methods such as single-molecule sequencing and nanopore sequencing are becoming mature. These advancements are set to enhance the accuracy and speed of sequencing, reducing the costs and time required for whole-genome sequencing. This progress will significantly promote the large-scale production and application of genomic data (Huang et al., 2018).
The application of artificial intelligence and machine learning in the analysis of genomic data is becoming increasingly widespread. As the volume of genomic data grows exponentially, traditional data analysis methods are becoming inadequate. Using artificial intelligence and machine learning technologies can efficiently parse these data, extracting more biological information and identifying potential therapeutic targets.
The integration of genomics with other omics technologies will also be a significant future direction (Gregory et al., 2019). For example, the combination of genomics with transcriptomics, proteomics, and metabolomics can reveal the molecular mechanisms of organisms under various physiological and pathological states more comprehensively, providing more precise guidance for disease diagnosis and treatment.
4.2 New technologies and methods in personalized cancer treatment
In recent years, a variety of new technologies and methods have emerged in the field of personalized cancer treatment, enhancing treatment efficacy and significantly reducing side effects, thus offering new hope to cancer patients.
Among these, genomic technologies are undoubtedly one of the most representative methods in personalized cancer treatment. By performing whole-genome sequencing on a patient's tumor tissue, doctors can precisely understand the genetic mutations present, allowing them to tailor personalized treatment plans. For instance, specific gene mutations may guide the selection of targeted drugs that act directly on cancer cells, achieving precise treatment effects.
Besides genomic technologies, immunotherapy has brought breakthroughs to personalized cancer treatment. By activating the patient's immune system to attack cancer cells, immunotherapy has become a lifesaver for many cancer patients. Particularly, therapies targeting specific tumor markers, such as CAR-T cell therapy and PD-1 inhibitors, have shown remarkable results in clinical trials.
4.3 Prospects of gene editing and gene repair technologies in personalized cancer treatment
The prospects for applying gene editing and gene repair technologies in personalized cancer treatment are broad and promising. These technologies, especially the CRISPR-Cas9 gene-editing system, have revolutionized cancer treatment (Mary et al., 2020). Gene editing can precisely target and modify specific genes within a patient’s body, correcting or eliminating mutations that cause cancer. For example, editing oncogenes or tumor suppressor genes can restore normal cell function and prevent cancer progression.
In personalized cancer treatment, gene editing technologies can tailor treatment plans based on a patient's genomic information. Editing a patient's own immune cells to more effectively recognize and attack cancer cells strengthens immunotherapy as a powerful weapon. Additionally, gene editing can be used to develop new cell therapies, such as CAR-T cell therapy, by modifying T cells to express specific cancer-fighting receptors.
Gene repair technologies focus on repairing damaged DNA, preventing or reversing the onset of cancer. In cancer treatment, gene repair can address hereditary diseases caused by genetic mutations, reducing cancer risk. Moreover, repairing DNA damage in tumor cells can inhibit their proliferation and spread, offering a new strategy for cancer treatment.
Although gene editing and gene repair technologies are still in the early stages of application in cancer treatment, continued improvements and in-depth clinical research are expected to bring more breakthroughs and progress in the future. These technologies hold the potential to be key in curing cancer, improving the quality of life and extending the lifespan of cancer patients.
5 Conclusion and Outlook
As technology continues to advance, genomics is gradually unveiling the profound mysteries of life, particularly in the field of cancer treatment, where its application prospects and significance are increasingly prominent. Personalized medicine, also known as precision medicine, is becoming an important direction in modern medical development. It emphasizes creating unique treatment plans based on the specific biological characteristics of each patient, aimed at improving treatment outcomes, reducing side effects, and enhancing the quality of life for patients (Janet et al., 2020).
Genomic technologies, especially whole-genome sequencing, provide strong support for the precise diagnosis and treatment of cancer. By conducting in-depth analysis of the genomic data from a patient's tumor tissue, doctors can accurately understand the genetic mutations within the patient, thus developing personalized treatment plans. This genomics-based precision treatment strategy not only improves treatment outcomes but also significantly reduces unnecessary side effects, bringing greater hope for survival to patients. Additionally, genomic technology offers new ideas for optimizing drug development and immunotherapy. By studying the functions of specific genetic mutations in depth, scientists can develop more precise and effective targeted drugs. The rise of immunotherapy has brought revolutionary changes to cancer treatment. By editing or regulating the patient's immune system, genomic technologies are expected to further enhance the effectiveness of immunotherapy, benefiting more patients.
However, despite significant achievements in personalized cancer treatment using genomics, numerous challenges remain. Further optimization of technology, enhanced integration and analysis of data, proper handling of ethical and privacy issues, and strengthened international cooperation are all critical directions for future research and development. Looking ahead, as genomic technologies continue to evolve and improve, personalized cancer treatment is expected to bring hope to more patients. We anticipate more breakthroughs and innovations in this field and believe that humanity will ultimately conquer this life-threatening adversary. In the journey towards this goal, genomics will continue to play an irreplaceable role, bringing a brighter future to cancer patients.
Aiello N.M., Maddipati R., Norgard R.J., Balli D., Li J., and Yuan S., 2018, EMT subtype influences epithelial plasticity and mode of cell migration, Dev. Cell, 45: 681-684.
https://doi.org/10.1016/j.devcel.2018.05.027
PMid:29920274 PMCid:PMC6014628
Besser J., Carleton H.A., Gerner-Smidt P., Lindsey R.L., and Trees E., 2018, Next-generation sequencing technologies and their application to the study and control of bacterial infections, Clin. Microbiol. Infect, 24: 335-341.
https://doi.org/10.1016/j.cmi.2017.10.013
PMid:29074157 PMCid:PMC5857210
Gregory L.A., Duncan R.M., Jill T., Heather A., Carleton M.P.H., Elizabeth B.N., Richard S.B., James E.P., and Marta G., 2019, Pathogen genomics in public health, N. Engl. J. Med., 381: 2569-2580.
https://doi.org/10.1056/NEJMsr1813907
PMid:31881145 PMCid:PMC7008580
Guthrie J.L., Strudwick L., and Roberts B., 2019, Whole genome sequencing for improved understanding of Mycobacterium tuberculosis transmission in a remote circumpolar region, Epidemiol Infect, 147: 188-188.
https://doi.org/10.1017/S0950268819000670
PMid:31364521 PMCid:PMC6518594
Gwinn M., MacCannell D., and Armstrong G.L., 2019, Next-generation sequencing of infectious pathogens, JAMA, 321: 893-894.
https://doi.org/10.1001/jama.2018.21669
PMid:30763433 PMCid:PMC6682455
Hill M.A., Alexander W.B., Guo B., Kato Y., Patra K., and O'Dell M.R., 2018, Kras and Tp53 mutations cause cholangiocyte- and hepatocyte-derived cholangiocarcinoma, Cancer Res., 78: 4445-4451.
https://doi.org/10.1158/0008-5472.CAN-17-1123
PMid:29871934 PMCid:PMC6097629
Huang S.J., Cai N.G., Pedro P.P., Shavira N., Wang Y., and Xu W.Y., 2018, Applications of support vector machine (SVM) learning in cancer genomics, Cancer Genomics & Proteomics, 15(1): 41-51.
https://doi.org/10.21873/cgp.20063
PMCid:PMC5822181
Janet P., Juan M.R.A., Josep S.P., Francesco R., Emilio C., Ferran S., and Laura I.F., 2020, The DisGeNET knowledge platform for disease genomics: 2019 update, Nucleic Acids Research, 48(1): 845-855.
MacCannell D., 2019, Platforms and analytical tools used in nucleic acid sequence-based microbial genotyping procedures, Microbiol. Spectr., 7(1): 5.
https://doi.org/10.1128/microbiolspec.AME-0005-2018
PMid:30737915
Mary J.G., Brian C., Mim H., Kristupas R., Fran M., Akhil K., Ayan B., Yunhai L., Dave R., Angela N.B., Zhu J.C., and David H., 2020, Visualizing and interpreting cancer genomics data via the Xena platform, Nature Biotechnology, 38: 675-678.
https://doi.org/10.1038/s41587-020-0546-8
PMid:32444850 PMCid:PMC7386072
Mook P., Gardiner D., and Verlander N.Q., 2018, Operational burden of implementing salmonella enteritidis and typhimurium cluster detection using whole genome sequencing surveillance data in England: a retrospective assessment, Epidemiol Infect, 146: 1452-1460.
https://doi.org/10.1017/S0950268818001589
PMid:29961436 PMCid:PMC9133683
Preethi K.A., Lakshmanan G., and Sekar D., 2021, Antagomir technology in the treatment of different types of cancer, Epigenomics, 13(7): 481-484.
https://doi.org/10.2217/epi-2020-0439
PMid:33719531
Satta G., Lipman M., Smith G.P., Arnold C., Kon O.M., and McHugh T.D., 2018, Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin. Microbiol Infect., 24: 604-609.
https://doi.org/10.1016/j.cmi.2017.10.030
PMid:29108952
Yamaguchi J., Yokoyama Y., Kokuryo T., Ebata T., and Nagino M., 2018, Cells of origin of pancreatic neoplasms, Surg Today, 48: 9-17.
https://doi.org/10.1007/s00595-017-1501-2
PMid:28260136
Yuan S., Norgard R.J., and Stanger B.Z., 2019, Cellular plasticity in cancer, Cancer Discov., 9(7): 837-851.
https://doi.org/10.1158/2159-8290.CD-19-0015
PMid:30992279 PMCid:PMC6606363
. PDF(374KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jiayao Zhou
Related articles
. Genomics
. Cancer
. Personalized therapy
. Precision medicine
. Genetic sequencing
Tools
. Email to a friend
. Post a comment