 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2023, Vol. 13, No. 1 doi: 10.5376/ijmms.2023.13.0001
Received: 27 Feb., 2023 Accepted: 06 Mar., 2023 Published: 10 Mar., 2023
Zhang Y.M., Yang Q.X., Zui L.F., Zhan Y., Yin Y.J., and Zhang J., 2023, Effects of saturated/unsaturated fatty acids on macrophage inflammatory status, International Journal of Molecular Medical Science, 13(1): 1-13 (doi: 10.5376/ijmms.2023.13.0001)
Studies have shown that fatty acids ingested into a body can affect the body's inflammatory status. In this study, eight kinds of fatty acids, which commonly presents in human daily diet, were added into bone marrow derived mouse microphages, respectively, then the mRNA abundance of the inflammation marker genes was analyzed to unveil the effects of fatty acids on macrophages inflammation. Results showed that saturated fatty acids, Palmitate acid (PA), Stearic acid (SA), Behenic acid (BA) and Lignoceric acid (LA), induced upregulation of TNFα, which indicated the macrophage became pro-inflammatory, and the effects of PA were highest. Unsaturated fatty acids, Palmitoleate acid (PO), Arachidonic acid (ARA), Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA), induced upregulation of IL10, which indicated the macrophage became anti-inflammatory, and the effects of DHA were the highest. Furthermore, added unsaturated fatty acids could reduce inflammatory states induced by PA, the effects of ARA were the most significant, followed by DHA. Western blot showed that the effects of PA, LA, ARA and DHA on macrophages inflammation were through NF-κB signaling pathway. In summary, saturated fatty acids PA, SA, BA and LA induce macrophages pro-inflammatory, while unsaturated fatty acid PO, ARA, EPA and DHA can alleviated macrophages inflammatory, with the best effects of ARA.
With the development of social economy, the problem of obesity is becoming more and more serious (Saltiel, 2016). Recent studies have shown that a variety of diseases closely related to obesity are related to chronic low-grade inflammation (CLI) caused by obesity (Amano et al., 2014). Chronic low-grade inflammatory response refers to a non-specific, chronic and persistent low-grade inflammatory pathological state. The chronic inflammatory response of the body is caused by immune cells infiltrating tissues and secreting inflammatory factors. Macrophages are the main cells that cause chronic low-grade inflammation in the body. Obesity in the body will cause more macrophages to infiltrate fat, muscle, liver and other tissues, inducing chronic low-grade inflammation (Amano et al., 2014). Macrophages can be in non-inflammatory state M0 in vivo, and under the stimulation of specific signals, they can be polarized into inflammatory state M1, the expression of tumor necrosis factor α (TNFα), interleukin 6 (IL6), inducible nitric oxide synthase (iNOS), CXC-motif chemokine ligand 1 (CXCL1) of genes related to inflammatory response increases, or they can be polarized into anti-inflammatory state M2. The expression of anti inflammatory gene type I arginase (Arginase1, Arg1), macrophage galactose-type lectin2 (Mgl2), interleukin 10 (IL10), mannose receptor gene 1 (Mrc1) increased (Wynn et al., 2013; Van et al., 2014; Boniakowski et al., 2017).
Various fatty acids in human diet not only serve as nutrients, but also as signal molecules to change the physiological state of the body. Animal experiments have shown that feeding ω-3 unsaturated fatty acids (DHA) to mice can significantly reduce chronic low-grade inflammation and improve insulin sensitivity (Calder, 2017). In vitro cell experiments showed that when macrophages were exposed to saturated fatty acids, macrophages could be polarized into M1-like cells, showing inflammation. However, exposure to unsaturated fatty acids causes macrophages to polarize into M2-like, showing an anti-inflammatory state, mediated by the NF-κB signaling pathway (Chan et al., 2015; Calder, 2017; Li et al., 2019). NF-κB is an important intracellular nuclear transcription factor involved in the expression and regulation of many genes. IκBα can inhibit NF-κB activity, so its expression level can reflect the activity of this signaling pathway (Zheng et al., 2016). The inflammatory genes involved in this study were all activated by NF-κB. It can be seen that fatty acids, as signaling substances, have an important effect on the inflammatory state of the body.
Similar reports have also been reported in China, but most of the studies were conducted using immortalized macrophage cell lines (Luo et al., 2015). Since the immortalized cell line has been artificially modified, it is different from the physiological state of macrophages. In this study, macrophages derived from the differentiation of mouse bone marrow cells were selected for primary culture, and eight fatty acids were studied in order to clarify their effects on the inflammatory state of macrophages, provide theoretical guidance for the diet of obese people, and lay a foundation for the molecular nutrition research of fatty acids.
1 Results and Analysis
1.1 Polarization of macrophages derived from murine bone marrow
In the absence of stimulatory factors, macrophages showed irregular morphology (Figure 1A), which was M0 type. Under the combined action of LPS and IFN-γ, macrophages became spherical in shape and enlarged in size (Figure 1B), and the mRNA abundance of iNOS increased by more than 10 000 times (Figure 1D), indicating that the cells underwent a typical inflammatory response and were polarized into M1 macrophages. Under the action of IL4, macrophages became elongated and dendritic (Figure 1C), and the mRNA abundance of Arg1 increased by more than 15 000 times (Figure 1E), indicating that the cells underwent a typical anti-inflammatory response and were polarized into M2 macrophages. These results indicate that the bone marrow-derived macrophages used in this study can respond to external inflammatory signals.
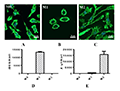 Figure 1 The morphologies and marker genes expression analysis of polarized macrophages Note: A-C The morphologies of unpolarized macrophages (M0), polarized macrophages (M1) and polarized macrophages (M2); D& E The mRNA abundance of iNOS and Arg1 in three types of macrophages |
1.2 Effects of saturated fatty acids on the inflammatory state of macrophages
After the addition of four saturated fatty acids, the expression levels of iNOS and TNFα were significantly upregulated (Figure 2A&B). Although the expression level of Arg1 did not change significantly (Figure 2C), the expression level of IL10 was significantly decreased (Figure 2D), indicating that the four saturated fatty acids could cause inflammation in macrophages. It produces M1-like macrophages. However, fatty acids with different carbon chain lengths had significant differences in the intensity of inflammatory response. Combined with the mRNA abundance changes of TNFα and IL10, 16-carbon palmitic acid (PA) caused the strongest inflammatory response. This conclusion was confirmed by enzymolinked immunoassay (ELISA), which measured the amount of TNFα secreted by M1-like macrophages into the medium (Figure 2E).
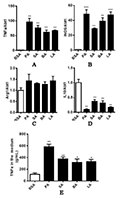 Figure 2 The effects of saturated fatty acids on inflammatory genes expression in mouse macrophages Note: A-D: The relative mRNA abundance of iNOS, TNFα, Arg1 and IL10 in macrophages after the administration of saturated fatty; E: The Elisa analysis of TNFα in macrophages medium after the administration of saturated fatty |
1.3 Effects of unsaturated fatty acids on the inflammatory state of macrophages
EPA and DHA caused a significant decrease in iNOS expression, while PO and ARA caused an insignificant decrease in iNOS expression (Figure 3A). The four unsaturated fatty acids all caused a downregulation of TNFα expression (Figure 3B), and an up-regulation of anti-inflammation gene Arg1 and IL10 expression (Figure 3C&D). It can be seen that the four unsaturated fatty acids not only do not cause inflammatory response of macrophages, but also can cause anti-inflammatory response to varying degrees, resulting in macrophages polarization into M2-like cells, among which DHA has the most significant effect. The above conclusions were confirmed by the amount of TNFα secreted by M2-like macrophages into the culture medium by ELISA (Figure 3E).
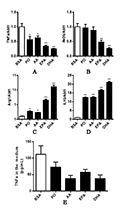 Figure 3 The effects of unsaturated fatty acids on inflammatory genes expression in mouse macrophages Note: A-D: The relative mRNA abundance of iNOS, TNFα, Arg1 and IL10 in macrophages after the administration of unsaturated fatty; E: The Elisa analysis of TNFα in macrophages medium after the administration of unsaturated fatty |
1.4 The alleviatory effects of unsaturated fatty acids on saturated fatty acid inflammation
In order to determine whether unsaturated fatty acids can relieve the inflammatory state of the body, saturated fatty acid PA was used in this study to stimulate macrophages. After changing the medium, an unsaturated fatty acid was added. 48 h later, the expression abundance of inflammatory marker genes iNOS, TNF-α and anti-inflammatory marker genes Arg1 and IL10 was analyzed by Real-time PCR. The results showed that the expression levels of inflammatory marker genes iNOS and TNF-α were significantly decreased (Figure 4A&B), ARA was significantly decreased, followed by DHA. The expression level of the anti-inflammatory marker gene Arg1 was increased (Figure 4C), but only the DHA group showed a significant difference. The expression of the anti-inflammatory marker gene IL10 was significantly increased (Figure 4D), among which the ARA group had the largest increase. Both unsaturated fatty acids can relieve the inflammatory response caused by PA, and the relieving ability is corresponding to the anti-inflammatory response ability caused by unsaturated fatty acids, among which ARA has the best relieving effect, followed by DHA. The amount of TNFα secreted by macrophages into the culture medium was measured by ELISA (Figure 4E), which confirmed the above conclusions.
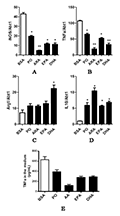 Figure 4 The alleviatory effects of unsaturated fatty acids on macrophages inflammation induced by PA Note: A-D: The relative mRNA abundance of iNOS, TNFα, Arg1 and IL10 in macrophages after the administration of fatty acids; E: The Elisa analysis of TNFα in macrophages medium after the administration of fatty acids |
1.5 Effects of fatty acids on NF-κB signaling pathway
In order to explore how the addition of fatty acids affects the NF-κB signaling pathway, the two saturated fatty acids PA and LA, which cause the most significant inflammatory reaction, and the two unsaturated fatty acids ARA and DHA, which have the most significant anti-inflammatory reaction, were selected for Western blot analysis of their effects on the expression level of IκBα after treatment (Figure 5A). The results showed that compared with the BSA control group (Figure 5B), PA and LA treatments significantly reduced the expression level of IκBα, increased the activity of NF-κB, and activated inflammation-related genes. ARA and DHA treatments increased the expression of IκBα and inhibited the activity of NF-κB, which affected the expression of inflammation-related genes. The results of this study indicated that the effects of four fatty acids on macrophages were related to the NF-κB signaling pathway. (Shanghai) Co., Ltd.
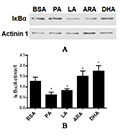 Figure 5 The effects of fatty acids on NF-κB signaling pathway Note: A: Effects of PA, ARA and DHA on the IκBα abundance in macrophages; B: Relative quantification analysis of the western blot results |
2 Discussion
Fatty acids are the most important molecules (nutrients) in the energy supply of all animals, and they are also synthetic precursors of eicosanoid acids (prostaglandins, hemoxane, and leukotrienes). However, in recent years, more and more evidence shows that fatty acids are involved in transcriptional regulation of gene expression. Based on these findings, a breakthrough has been made in the study of the effects of fatty acids on inflammatory states in animals. DHA has been reported as the object of most studies, from feeding in living animals (Bascoul-Colombo et al., 2008), to intracellular signaling pathways (Hernandez-Rodas et al., 2017), and the molecular mechanism of action. DHA is believed to inhibit inflammatory gene-related nuclear transcription factor NF-κB and activate anti-inflammatory transcription factor peroxidase proliferator receptor γ (PPARγ) by altering the composition of phospholipid fatty acids in cell membranes and disrupting lipid rafts (Calder, 2017). These studies help to understand the mechanism behind the effects of DHA rich deep-sea fish oil on human health, and suggest that fatty acids, as signaling molecules, have complex effects on human health.
But the effect of most fatty acids on inflammatory states in animals has not been known. In this study, 8 kinds of common fatty acids were selected, and macrophages cultured in mouse primary culture were taken as the research object to analyze the effects of these 8 kinds of fatty acids on the inflammatory state of macrophages, and strive to find regular conclusions. The 16-carbon saturated fatty acid PA involved is mainly found in tallow oil and palm oil, the 18-carbon stearic acid SA is mainly found in animal fat and cocoa butter, the 20-carbon BA is abundant in soybean oil and sesame oil, and the 22-carbon LA is found in peanut oil, all of which are common fatty acids in Chinese recipes, but there has been no previous study on its correlation with inflammation. The unsaturated fatty acids involved in this study, PO, are found in milk fat, fish oil, and algae foods. The health benefits of ARA, EPA and DHA have been known for a long time and are found in egg yolks and deep-sea fish. In this study, they were selected as representatives of unsaturated fatty acids and compared with saturated fatty acids to study their effects on the inflammatory state of macrophages. The results showed that the four saturated fatty acids selected in this study can cause macrophage inflammatory response, while the unsaturated fatty acids can cause macrophage anti-inflammatory response, but the amplitude is much smaller than the typical inflammatory/anti-inflammatory response induced by LPS and IFN-γ/IL4. Fatty acid stimulation led to the up-regulation of inflammatory marker genes iNOS by 40 to 50 times, while LPS and IFN-γ stimulation led to the up-regulation of inflammatory marker genes iNOS by more than 10 000 times. The anti-inflammatory marker Arg1 was upregulated by 4-12 fold by unsaturated fatty acids, while IL4 was upregulated by more than 15 000 fold. The result is in line with our expectation. LPS is a thick layer of lipid-like polysaccharide located in the outermost layer of the cell wall of Gram-negative bacteria (Kim et al., 2016). It stimulates the immune system and triggers an acute inflammatory response, usually in bacterial infections. When fatty acids enter the body, the stimulation of immune formation is slow and cumulative. For example, people who eat a high-heat diet for a long time often have a low degree of chronic inflammation.
In addition, the intensity of inflammatory/anti-inflammatory response induced by different fatty acids was different under the premise of consistent stimulus concentration. The 16-carbon saturated fatty acid PA caused the highest intensity of inflammatory response in the four inflammatory/anti-inflammatory marker genes. The intensity of inflammatory response caused by the other 3 saturated fatty acids was similar, and lower than PA. The anti-inflammatory effect caused by unsaturated fatty acids, ARA of carbon 20 is the strongest, EPA of carbon 22 and DHA of carbon 24 are similar, and PO of carbon 16 is the weakest. Therefore, there was no significant correlation between fatty acid-induced inflammatory/anti-inflammatory response and carbon chain length.
In order to compare the protective effect of unsaturated fatty acids on the body in anti-inflammatory response, saturated fatty acids PA was used to stimulate macrophages to produce inflammatory response, and then unsaturated fatty acids were added. It was found that all four kinds of unsaturated fatty acids could alleviate the inflammatory response caused by PA to a certain extent. This remission was correlated with the anti-inflammatory response induced by unsaturated fatty acids, with ARA having the strongest protective effect, EPA and DHA having similar effects, and PO having the weakest effect. ARA belongs to the ω-6 polyunsaturated fatty acids, which are direct precursors to the synthesis of eicosane derivatives such as prostaglandins, thromboxanes and leukotrienes, and are essential for human health. In addition, ARA has multiple physiological functions, such as promoting the growth of skeletal muscle (Trappe and Liu, 2013) and being closely associated with Alzheimer's disease (Schaeffer et al., 2009; Amtul et al., 2012). There is no consensus on the effect of ARA on anti-inflammatory response and on the health care of chronic low-grade inflammatory response. There have been several reports that when a certain amount of ARA is supplemented daily (between 840 and 2 000 mg) for 50 days, the inflammatory marker molecules in vivo are not changed (Nelson et al., 1997; Harris et al., 2009). However, a study at Baylor College of Medicine found that 1 000 mg of ARA supplementation daily for 50 days reduced the levels of inflammatory marker molecules IL1 and IL6 and increased the levels of anti-inflammatory marker molecules TNFβ in the blood (Ferrucci et al., 2006). The studies show different results, which may have something to do with the organization, conduct and population. However, in this study, cultured macrophages were used as materials to confirm the effect of ARA on relieving chronic low-grade inflammatory response. The ω-3 fatty acids EPA and DHA have been widely recognized for their role in reducing chronic low-grade inflammation. However, compared with the two, ARA has a wider range of sources, relatively low price, and has better application prospects.
In conclusion, saturated fatty acids selected in this study can cause inflammatory responses of macrophages to varying degrees, while unsaturated fatty acids can cause anti-inflammatory responses of macrophages. This suggests that we should try to reduce the intake of saturated fatty acids and moderately increase the intake of unsaturated fatty acids when choosing food for people suffering from low-grade chronic inflammatory reaction, which will help alleviate low-grade chronic inflammatory reaction and improve their health.
3 Materials and Methods
3.1 Materials
C57BL/6N mice, male, about 6 weeks old, were purchased from the Genome Resource Center of Cambridges-SU University and kept in the Laboratory Animal Center (SPF) of Jiaxing University School of Medicine. Eight fatty acids (Table 1), macrophage colony-stimulating factor (M-CSF) (Sigma Corporation, USA); R/MINI1 640 culture-medium, fetal bovine serum (FBS) and non-essential amino acids (Gibco, USA), β-mercaptoethanol (Amresco, UK); TNFα Elisa kit, Nanjing Jiancheng Biotechnology Co., LTD.
 Table 1 Saturated/unsaturated fatty acids used in this study |
3.2 Isolation and culture of mouse macrophages derived from bone marrow
The mice were killed by cervical vertebra dislocation. Two hind legs were removed. After the muscle was removed, the leg bones were cut at both the upper and lower positions of the knee joint, and the bone marrow cells were collected by centrifugation at a temperature of 12 000 r/min at 4°C for 10 s. The leg bones were discarded and 1 mL of R/MINI1 640 medium preheated at 37°C was added to the precipitate. The precipitate was suspended, the massive tissue was removed, and then centrifuged again at 12 000 r/min at 4°C. The supernatant was abandoned and re-suspended precipitated with R/MINI1 640 medium (10% FBS, 1% non-essential amino acids, 0.5% β-mercaptoethanol), counted, and inoculated into six-well plates with about 1×106 cells per well. After adherent for 2 h, the medium was changed and R/MINI1 640 complete medium was added with macrophage colony stimulating factor (M-CSF, 50 ng/mL). The medium was cultured in an incubator with 5% CO2, saturated humidity and 37°C every 3 days. After 7 days, the cell morphology was observed under laser focusing microscope.
3.3 Polarization of macrophages
Macrophages were divided into three groups. The first group did not add any reagent, the second group added stimulatory factor LPS (50 μg/mL) and IFN-γ (5 ng/mL), and the third group added stimulatory factor IL4 (10 ng/mL). The macrophages were cultured in 5% CO2, saturated humidity and 37°C for 2 days. Cytoskeleton was stained with Phalloidin and cell morphology was observed under laser confocal microscope.
3.4 Stimulating macrophages with fatty acids
Macrophages were uniformly inoculated into culture dishes with a cell density of 70-80%. Eight kinds of fatty acids (0.5 mmol /L) were added to macrophages, respectively. With BSA as the control, the cells were collected after 2 days of culture in 5% CO2 incubator at 37°C.
3.5 Detecting inflammatory response marker genes by Real-time PCR
Total RNA was extracted by Trizol method and cDNA was synthesized by SuperScript II. The Real-time PCR reaction procedure was as follows: 95°C predenaturation for 20 s, 95°C denaturation for 1 s, 60 ℃ extension for 20 s, 40 cycles, 72°C 10 min. With Abt1 as the reference gene, △△Ct calculated that the target gene was relatively rich in expression. The Real-time PCR primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Table 2).
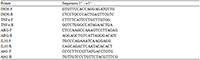 Table 2 Primers used for real-time quantitative PCR analysis |
3.6 The changes of IκBα detected by Western blot
When the cell density was appropriate, the cell medium was removed, and the cells were washed twice with cold PBS. After adding an appropriate amount of RIPA lysate, the culture plate was placed in the ice domain, the cells were scraped off, centrifuged at 4°C at 13 000 r/min for 6 min, the supernatant was absorbed into 1.5 mL enzyme-free centrifuge tube, the protein loading buffer was added, mixed and boiled for 10 min for denaturation. Take supernatant for quantification. The denatured proteins were subjected to 10% SDS-PAGE electrophoresis. The protein was transferred to 0.45 μm PVDF membrane, sealed with 5% skim milk powder and shaker at 37°C for 60 min. The primary antibody (IκBα and Actinin1) was incubated overnight at 4°C, washed three times with TBST for 5 min each time, and the secondary antibody was added and incubated in the shaker at room temperature for 1 h, washed three times with TBST for 5 min each time. After being developed by the developer, the gel imaging system takes photos and analyzes the gray value of each band.
3.7 The secretion of TNFα detected by Elisa
The secretion of TNFα was detected according to the instructions of Elisa kit. The description is as follows: add 50 μL standard diluent to each well (not to blank well), then add 50μL biotin antigen working solution, mix gently, seal the film, and incubate at 37°C for 30 min. Remove the membrane, discard the liquid, add 300μL washing solution, stand for 30 s, then discard the liquid, repeat 5 times, add 50 μL avidin-HRP, mix, incubate at 37°C for 30 min. Repeat the washing process. Add 50 μL color developing agent A, then add 50 μL color developing agent B, mix well, and develop color at 37°C for 10 min away from light. The reaction was terminated by adding 50 μL termination solution to each well. The absorbance (OD value) was measured at 450 nm wavelength with blank hole zeroing. The standard curve was drawn with logistic curve model in ELISAcalc software. Use the same method to measure the absorbance of the sample and calculate the corresponding concentration.
3.8 Stimulating PA-stimulated macrophages with unsaturated fatty acids
PA was added to macrophages and cultured in a 5% CO2 incubator at 37°C. After 2 days of culture, the cell status was observed. Fresh medium was changed and 4 kinds of unsaturated fatty acids were added. As control, Bsas were cultured in an incubator with 5% CO2, saturated humidity and 37°C. After 2 days, the cells were collected and the expression level of marker genes was verified by Real-time PCR.
3.9 Statistical analysis of data
The experiment was repeated three times and the results were represented by SEM. SPSS17.0 software was used for descriptive analysis and t test analysis, and the test level α=0.05 (Mean ± SEM, n=3; *: P<0.05; **: P< 0.01).
Authors’ contributions
ZYM was mainly responsible for cell experiment and paper writing. YQX was responsible for Real-time PCR detection and Elisa detection. ZLF was responsible for Western blot detection and data analysis. YYJ and ZJ were the project leaders, mainly responsible for experimental design, experimental guidance and paper modification. All authors read and approved the final manuscript.
Acknowledgments
This research was supported by the Natural Science Foundation of Zhejiang Province (LY20C170003).
Amano S.U., Cohen J.L., Vangala P., Tencerova M., Nicoloro S.M., Yawe J.C., Shen Y., Czech M.P., and Aouadi M., 2014, Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation, Cell Metabolism, 19(1): 162-171.
https://doi.org/10.1016/j.cmet.2013.11.017
Amtul Z., Uhrig M., Wang L., Rozmahel R.F., and Beyreuther K., 2012, Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer's disease: structural insight, Neurobiology of Aging, 33(4): 831.e21-31
https://doi.org/10.1016/j.neurobiolaging.2011.07.014
Bascoul-Colombo C., Guschina I.A., Maskrey B.H., Good M., O'Donnell V.B., and Harwood J.L., 2016, Dietary DHA supplementation causes selective changes in phospholipids from different brain regions in both wild type mice and the Tg2 576 mouse model of Alzheimer's disease, BBA Molecular and Cell Biology of Lipids, 1861(6): 524-537
https://doi.org/10.1016/j.bbalip.2016.03.005
Boniakowski A.E., Kimball A.S., Jacobs B.N., Kunkel S.L., and Gallagher K.A., 2017, Macrophage-mediated inflammation in normal and diabetic wound healing, The Journal of Immunology, 199(1): 17-24
https://doi.org/10.4049/jimmunol.1700223
Calder P.C., 2017, Omega-3 fatty acids and inflammatory processes: from molecules to man, Biochemical Society Transactions, 45(5): 1105-1115
https://doi.org/10.1042/BST20160474
Chan K.L., Pillon N.J., Sivaloganathan D.M., Costford S.R., Liu Z., Théret M., Chazaud B., and Klip A., 2015, Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK), Journal of Biological Chemistry, 290(27): 16979-16988
https://doi.org/10.1074/jbc.M115.646992
Ferrucci L., Cherubini A., Bandinelli S., Bartali B., Corsi A., Lauretani F., Martin A., Andres-Lacueva C., Senin U., and Guralnik J.M., 2006, Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers, Journal of Clinical Endocrinology & Metabolism, 91(2): 439-446
https://doi.org/10.1210/jc.2005-1303
Harris W.S., Mozaffarian D., Rimm E., Kris-Etherton P., Rudel L.L., Appel L.J., Engler M.M., Engler M.B., and Sacks F., 2009, Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the american heart association nutrition subcommittee of the council on nutrition, physical activity, and metabolism; council on cardiovascular nursing; and council on epidemiology and prevention, Circulation, 119(6): 902-907
https://doi.org/10.1161/CIRCULATIONAHA.108.191627
Hernández-Rodas M.C., Valenzuela R., Echeverría F., Rincón-Cervera M.Á., Espinosa A., Illesca P., Muñoz P., Corbari A., Romero N., Gonzalez-Mañan D., and Videla L.A., 2017, Supplementation with docosahexaenoic acid and extra virgin olive oil prevents liver steatosis induced by a high-fat diet in mice through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and NF-κB downregulation, Molecular Nutrition & Food Research, 61(12)
https://doi.org/10.1002/mnfr.201700479
Kim Y., Lee E.J., Jang H.K., Kim C.H., Kim D.G., Han J.H., and Park S.M., 2016, Statin pretreatment inhibits the lipopolysaccharide-induced epithelial-mesenchymal transition via the downregulation of toll-like receptor 4 and nuclear factor-κB in human biliary epithelial cells, Journal of Gastroenterology and Hepatology, 31(6): 1220-1228
https://doi.org/10.1111/jgh.13230
Li M.j., Sun Q., Wen X.N., Lu C., and Ruan G.H., 2019, Effects of Four Kinds of Drinking Water on Innate Immunity and T-lymphocyte Subsets in Mice, Jiyin Zuxue Yu Yingyong Shengwuxue (Genomics and Applied Biology), 38(4): 1898-1906
Luo W.J., Sui Y.H., Lian M., and Hua J., 2015, Effects of different fatty acids on macrophage M1/M2 polarization, Changweibing Xue (Chinese Journal of Gastroenterology), 20(1): 24-28
Nelson G.J., Schmidt P.C., and Corash L., 1991, The effect of a salmon diet on blood clotting, platelet aggregation and fatty acids in normal adult men, Lipids, 26(2): 87-96
https://doi.org/10.1007/BF02544000
Saltiel A.R., 2016, New therapeutic approaches for the treatment of obesity, Science Translational Medicine, 8(323): 323rv2
https://doi.org/10.1126/scitranslmed.aad1811
Schaeffer E.L., Forlenza O.V., and Gattaz W.F., 2009, Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease, Psychopharmacology, 202(1-3): 37-51
https://doi.org/10.1007/s00213-008-1351-0
Trappe T.A., and Liu S.Z., 2013, Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise, Journal of Applied Physiology, 115(6): 909-919
https://doi.org/10.1152/japplphysiol.00061.2013
Van O.E., Laoui D., Keirsse J., Van G.J.A., and Sarukhan A., 2014, Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues, Frontiers in Immunology, 5: 127
https://doi.org/10.3389/fimmu.2014.00127
Wynn T.A., Chawla A., and Pollard J.W., 2013, Macrophage biology in development, homeostasis and disease. Nature, 496(7446): 445-455
https://doi.org/10.1038/nature12034
Zheng B., Wen Z.S., Huang Y.J., Xia M.S., Xiang X.W., and Qu Y.L., 2016, Molecular weight-dependent immunostimulative activity of low molecular weight chitosan via regulating NF-κB and AP-1 signaling pathways in RAW264.7 macrophages, Marine Drugs, 14(9): 169
. PDF(419KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Yaming Zhao
. Qiuxian Yang
. Lingfeng Zuo
. Zhan Yang
. Yajun Yin
. Jin Zhang
Related articles
. Fatty acid
. Macrophage
. NF-κB signaling pathway
Tools
. Email to a friend
. Post a comment