Review and Progress
Applications of Gene Editing and Gene Therapy in the Treatment of Genetic Disorders 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 1 doi: 10.5376/ijmms.2024.14.0001
Received: 15 Nov., 2023 Accepted: 20 Dec., 2023 Published: 01 Jan., 2024
Cai X.P., 2024, Applications of gene editing and gene therapy in the treatment of genetic disorders, International Journal of Molecular Medical Science, 14(1): 1-7 (doi: 10.5376/ijmms.2024.14.0001)
Gene editing and gene therapy are advanced biotechnologies that have gained significant attention in recent years, holding enormous potential for the treatment of genetic disorders. This review provides an overview of the applications of gene editing and gene therapy in the treatment of genetic diseases. It introduces the concept of gene editing and places a specific emphasis on the application of the CRISPR-Cas9 system in genetic disease research and therapy. Furthermore, the review outlines the principles and methods of gene therapy, including gene replacement, gene correction, and gene silencing, discussing their applications in the treatment of genetic disorders. By comparing the advantages and limitations of both gene editing and gene therapy strategies, including technical constraints, safety concerns, and ethical considerations, this review summarizes the current applications of gene editing and gene therapy in the treatment of genetic diseases and provides insights into future trends. The aim of this review is to offer a reference and guidance for research and clinical applications of gene editing and gene therapy in the field of genetic disease treatment.
Genetic diseases are diseases caused by abnormal gene or chromosome mutations, which impose a significant burden on both patients and society as a whole. Traditional treatment methods primarily focus on alleviating disease symptoms and managing associated complications, but they rarely have the ability to cure the disease itself. However, with the rapid development of gene editing and gene therapy technologies, new treatment strategies are emerging. Gene editing technology, especially the emergence of the CRISPR-Cas9 system, provides an unprecedented option for the treatment of genetic diseases (Hu, 2022). Gene editing allows for the precise modification of abnormal genes within a patient's body, effectively correcting genetic defects and restoring normal gene function. Compared to traditional gene therapy methods, gene editing offers higher precision, a wider range of applications, and greater treatment efficacy. Furthermore, gene editing can also be used to repair a patient's hematopoietic stem cells ex vivo, offering hope incurable by traditional methods.
In addition to gene editing, gene therapy is also an important direction in the field of genetic disease treatment (Yang, 2020). Gene therapy uses delivery vectors to introduce normal genes into patients' bodies to repair or replace abnormal genes. This method has made some important breakthroughs in the treatment of monogenic genetic diseases, such as the cure of some rare genetic diseases through gene replacement therapy. In addition, gene therapy can also regulate gene expression through strategies such as stem cells and gene silencing, effectively controlling the development and progression of hereditary diseases.
Although the application of gene editing and gene therapy has shown great potential and hope in the treatment of genetic diseases, it also faces some challenges. Technical limitations, security issues, and ethical and legal considerations are currently key issues that need to be addressed. Therefore, further research and standardized regulation are essential.
This review will systematically review the application of gene editing and gene therapy in the treatment of genetic diseases, explore the principles, methods, and key application cases of these emerging technologies, and compare their advantages and current challenges. This review paper can provide comprehensive understanding for researchers and clinical doctors, and point out the direction for future research and practice in the treatment of genetic diseases.
1 Application of Gene Editing in the Treatment of Hereditary Diseases
1.1 Definition of gene editing
Gene editing refers to the technique of accurately modifying and adjusting the genome of an organism through deliberate human intervention. The main principle is to introduce specific nucleases or protein tools to interact with the target DNA sequence, achieving operations such as DNA mutation, insertion, or deletion. Among them, the most widely used gene editing technology is the CRISPR-Cas9 system. This system utilizes Cas9 protein binds to specific guiding RNA (gRNA) to form complexes for precise identification and binding of target DNA sequences, thereby triggering the process of genome editing. In addition to the CRISPR-Cas9 system, there are other gene editing techniques such as zinc finger nucleases (ZFNs) and deoxyribonucleases (TALENs) that can also achieve precise genome modifications. Different gene editing techniques differ in terms of target selection, editing efficiency, and specificity.
1.2 Application of gene editing in the treatment of genetic diseases
1.2.1 Application in the treatment of monogenic genetic diseases
Monogenic genetic diseases, caused by mutations in a single gene, have found new hope in the application of gene editing technologies. The primary tool for gene editing is the CRISPR-Cas9 system, which allows precise modification of the genome to restore normal function. Gene editing can be employed to correct disease phenotypes by repairing mutated genes. This approach is applicable to specific types of mutations,, such as point mutations, insertions, and deletions. By introducing a repair template, gene editing can restore the normal sequence of genes and repair the functionality of disease-associated genes. For instance, in the context of cystic fibrosis, a common monogenic disease, gene editing techniques have been used to repair the CFTR gene. Researchers use the CRISPR-Cas9 system to precisely cleave the mutated gene sequence and introduce a normal repair template into cells through homologous recombination. This repair method has demonstrated feasibility and effectiveness in cell and animal models, providing a new avenue for gene therapy in cystic fibrosis.
For gene mutations that cannot be repaired, gene editing technologies can facilitate gene replacement, which involves inserting normal gene sequences into damaged gene sites to restore their normal function. This method is applicable to certain monogenic genetic diseases, such as spinal muscular atrophy. For instance, some studies have utilized gene editing techniques to insert the normal SMN1 gene sequence into the genome of patients with spinal muscular atrophy. This gene replacement approach has been shown to increase the levels of SMN protein in patients, thereby improving the symptoms of the disease.
Gene editing technology can also be employed for gene silencing, involving the precise cleavage and disruption of the sequence of detrimental genes to inhibit their function. This method is applicable to certain monogenic genetic diseases, such as fragile X syndrome. For example, fragile X syndrome is caused by an expansion mutation of the FMR1 gene on the X chromosome (Figure 1). In the treatment of fragile X syndrome, researchers have utilized the CRISPR-Cas9 system for gene silencing targeting the FMR1 gene (Liao et al., 2022). By accurately cleaving the sequence of this gene, the production of harmful proteins can be reduced, thereby alleviating the symptoms.
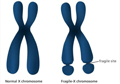 Figure 1 Differences between fragile X syndrome X chromosome and normal chromosome |
1.2.2 Application in the treatment of polygenic genetic diseases
Gene editing technology has demonstrated significant potential in the treatment of polygenic genetic diseases. Polygenic genetic diseases are disorders caused by mutations in multiple genes, such as hereditary heart disease, chromosomal abnormality syndromes and so forth. Gene editing can be utilized to repair mutations in multiple genes, in order to restore the functionality of genes associated with polygenic genetic diseases. By introducing multiple repair templates, it is possible to simultaneously correct mutations in different genes. This approach can be applied in certain polygenic genetic diseases, such as hereditary heart disease (Zhang et al., 2020).
Gene editing technology can also restore the normal function of genes associated with polygenic genetic diseases by replacing mutations in multiple genes. By simultaneously inserting multiple normal gene sequences, it is possible to ameliorate the pathological consequences of polygenic mutations. This method is applicable to polygenic genetic diseases such as chromosomal abnormality syndromes.
Gene editing technology can also be employed to regulate the expression levels of multiple genes. By precisely editing the regulatory sequences of genes, it is possible to increase or decrease the expression of multiple genes, thereby influencing disease-relevant signaling pathways. This method can be applied in certain polygenic genetic diseases, such as polygenic tumors.
2 Gene Therapy in the Treatment of Genetic Diseases
2.1 Definition of gene therapy
Gene therapy is a medical technique aimed at treating or preventing diseases by directly intervening in the human genome. It involves the introduction, modification, or deletion of gene sequences into the cells or tissues of patients to repair or alter abnormal or missing gene functions. The goal of gene therapy is to adjust or repair genes, restoring mutated genes or supplementing missing or abnormal genes to achieve therapeutic effects. The potential of this treatment lies in its ability to fundamentally impact the occurrence and progression of diseases. Gene therapy can take various forms, including gene replacement therapy, gene editing therapy, gene enhancement therapy, gene silencing therapy, and more.
2.2 Application of gene therapy in the treatment of genetic diseases
2.2.1 Application in the treatment of monogenic genetic diseases
Gene therapy has made significant strides in the treatment of monogenic genetic diseases (Zhou et al., 2020). Gene replacement therapy aims to replace defective genes with normal gene sequences to restore normal gene function. This method can be achieved by introducing normal genes into the cells or tissues of patients. For instance, for patients with cystic fibrosis (Figure 2), normal CFTR genes can be introduced into lung cells to restore the function of chloride ion channels.
 Figure 2 Cystic fibrosis |
Gene editing therapy involves directly modifying the patient's gene sequence to correct mutations, insertions, deletions, and other genetic abnormalities. This method utilizes tools such as CRISPR-Cas9 to perform precise gene editing targeted at specific gene mutations. For example, gene editing therapy can be employed to repair mutated genes in hematopoietic stem cells of patients with blood disorders, thereby correcting abnormalities in hemoglobin.
In some monogenic genetic diseases, patients may experience a deficiency or insufficient functionality of normal genes. Gene enhancement therapy aims to increase the expression levels of normal genes to achieve therapeutic effects. This method can be implemented by delivering additional normal genes into the patient's body. For instance, through gene enhancement therapy, it is possible to increase the expression of proteins associated with depression-related genes to improve symptoms.
2.2.2 Application in the treatment of polygenic genetic diseases
Gene therapy also holds promise in the treatment of polygenic genetic diseases, but compared to single gene inherited diseases, poses more challenges, and research is still in its early stages. Currently, the applications in the treatment of polygenic genetic diseases primarily involve gene silencing therapy, gene regulation therapy, and combination therapy strategies.
Gene silencing therapy aims to reduce or eliminate the adverse effects on health by inhibiting the overexpression or production of harmful genes. This can be achieved through RNA interference techniques, such as siRNA or miRNA. By selectively inhibiting the expression of specific genes, it is possible to intervene in the occurrence and progression of polygenic genetic diseases.
Polygenic genetic diseases typically involve aberrant regulation of multiple genes. The goal of gene regulation therapy is to restore a normal gene expression pattern by adjusting the balance of the patient's intracellular gene regulatory network. This may involve the use of gene expression regulatory factors (such as transcription factors or nucleases) to modulate the expression levels of relevant genes.
The treatment of polygenic genetic diseases often requires the integration of multiple therapeutic strategies. Gene therapy can be combined with other treatment modalities, such as drug therapy, gene editing, or stem cell transplantation, to achieve enhanced therapeutic outcomes. By combining multiple treatment strategies, a more comprehensive intervention in the development and symptoms of polygenic genetic diseases can be achieved.
3 Comparison between Gene Editing and Gene Therapy
3.1 Advantages and limitations in treating genetic diseases
The advantages of gene editing include precision; gene editing technologies (such as CRISPR-Cas9) can precisely modify DNA sequences, accurately correcting genetic mutations in patients and restoring normal gene function. Its therapeutic potential is significant; gene editing can potentially cure genetic diseases by repairing or correcting mutations present in the patient's DNA, restoring normal gene expression and function (Li et al., 2017). In terms of persistence, gene editing technology can induce enduring genetic changes in treated cells, which can be passed on to descendant cells, resulting in a long-term therapeutic effect.
The limitations of gene editing include safety concerns. Gene editing technology is still in the developmental stage, and unexpected side effects and safety issues, some of which may be unpredictable, could arise. Delivery challenges exist as the precise delivery of editing tools to specific tissues or organs in patients remains a challenge, necessitating effective delivery systems and strategies. In terms of ethical and moral considerations, gene editing involves the direct manipulation of human genes, sparking discussions on ethical and moral issues such as human genetic modification and genetic editing of embryos.
The advantages of gene therapy include deliverability (Li et al., 2019). Gene therapy utilizes vectors (such as viruses or plasmids) to introduce normal genes into the patient's body, ensuring accurate delivery to the cells or tissues requiring treatment. With a variety of treatment strategies, gene therapy can intervene in genetic diseases by enhancing the expression of normal genes, suppressing the expression of harmful genes, or supplementing missing genes. Successful cases already exist; in treating certain genetic diseases, gene therapy has achieved significant success, such as in the treatment of some immunodeficiency disorders and retinal diseases.
The limitations of gene therapy include clinical application restrictions. Currently, the clinical application of gene therapy is still subject to certain limitations, such as unstable treatment effects and the need for further validation of sustained efficacy. There are concerns about immune reactions and safety issues, the introduction of exogenous genes and vectors may trigger immune responses and safety issues, leading to treatment failure or adverse reactions. Cost is also a factor, the preparation and delivery costs of gene therapy are relatively high, limiting its feasibility for widespread application.
Overall, both gene editing and gene therapy hold promise and face challenges as methods for treating genetic diseases. With continuous technological advancements and in-depth research on their safety and efficacy, these two approaches are expected to offer broader prospects for the treatment of genetic diseases in the future.
3.2 Criteria for selection and evaluation in different genetic diseases
In selecting and assessing the application of gene editing and gene therapy methods for different genetic diseases, the following are common criteria. Regarding selection criteria, the first consideration is the characteristics of the disease, understanding the types of genetic mutations, the pathogenic mechanisms, and the severity of the disease. This helps determine whether gene editing or gene therapy can correct or alleviate the pathological processes caused by the genetic mutations. Additionally, considerations must include the accessibility of the target cells or tissues, determining whether gene editing or gene therapy techniques can effectively deliver to the specific cells or tissues requiring treatment. Assessing the therapeutic potential involves evaluating the efficacy and feasibility of gene editing or gene therapy methods in correcting genetic mutations, including theoretical effectiveness and pre-clinical study results. In the selection of competitive treatment methods, other possible treatments such as drug therapy or gene replacement therapy should be considered, evaluating the comparative advantages of gene editing or gene therapy against alternative approaches.
In terms of evaluation criteria, the first consideration is efficacy and safety, assessing the method's impact on patients' disease symptoms, survival rates, and quality of life, as well as potential treatment risks and adverse reactions. The second consideration is persistence, examining whether the treatment effects can be sustained and whether repeated treatments are necessary. Feasibility and cost-effectiveness should also be taken into account, considering the feasibility of the treatment method, including the preparation and delivery of the treatment plan, and evaluating its cost-effectiveness compared to other treatment methods. Additionally, there is a need to assess the ethical and moral issues inherent in the treatment method (Wang et al., 2021), such as the uncertainty of potential genetic editing or modification impacts on future generations and the potential negative effects on specific ethnic or social groups.
The above criteria are for reference only and are not exhaustive. In specific clinical applications, researchers and physicians need to comprehensively consider factors such as disease characteristics, treatment goals, safety, feasibility, and patient needs to make personalized choices and assessments. Additionally, ongoing clinical and scientific research is crucial for continually refining method selection and evaluation criteria.
3.3 Complementarity in treating genetic diseases
Gene editing and gene therapy complement each other in treating genetic diseases, offering mutual supplementation and synergistic effects at different levels. In terms of the scope of target diseases, gene editing and gene therapy exhibit complementarity in addressing different types of genetic mutations and disease mechanisms. Gene editing can precisely correct genetic mutations by modifying DNA sequences, particularly applicable to monogenic genetic diseases. On the other hand, gene therapy can intervene in various types of genetic diseases through methods such as delivering normal genes or suppressing the expression of harmful genes.
Gene editing and gene therapy complement each other in terms of treatment timing. Gene editing typically requires intervention in the early stages of embryos or stem cells to correct genetic mutations that occur early in the patient's body. In contrast, gene therapy can be performed at any point after the patient's birth, even when the disease has already developed and symptoms have appeared.
Gene editing and gene therapy possess complementary tissue and organ integration capabilities. Gene editing can achieve systemic efficacy by selectively editing problematic genes as needed. On the other hand, gene therapy can target specific tissues and organs, achieving targeted efficacy by selectively delivering corrective factors.
Gene editing and gene therapy exhibit complementarity in terms of the persistence of treatment effects. Gene editing can maintain enduring genetic changes in cells and descendants, providing long-term therapeutic effects (Zhu et al., 2019). Meanwhile, gene therapy can achieve long-term therapeutic effects through sustained gene expression or maintaining stable levels of corrective factors.
In summary, gene editing and gene therapy have complementary advantages in treating genetic diseases. Therefore, in specific clinical applications, a flexible combination of gene editing and gene therapy can be chosen based on factors such as the type of disease, treatment goals, and technical feasibility to maximize treatment effectiveness and provide personalized and comprehensive treatment plans for patients.
4 Summary and Outlook
Gene editing and gene therapy represent cutting-edge technologies for the treatment of genetic diseases, holding revolutionary potential in genetic modification and intervention. Gene editing, through direct modification of DNA sequences, can precisely correct genetic mutations, potentially curing genetic diseases. Gene therapy, on the other hand, involves delivering normal genes into the patient's body or suppressing the expression of harmful genes to block or improve the course of the disease. Both technologies have significant and undeniable potential in the treatment of genetic diseases.
In terms of clinical applications, gene editing and gene therapy have made significant strides. Clinical trials for some genetic diseases, such as cystic fibrosis and hereditary retinal disorders, have been conducted using gene editing or gene therapy, yielding promising results. These studies have laid the groundwork for further exploration of the clinical applications of gene editing and gene therapy.
In the future, the application of gene editing and gene therapy in the treatment of genetic diseases will face critical challenges and directions for development. Firstly, there is a need to enhance the precision, efficiency, and safety of gene editing techniques, reducing non-specific editing and potential side effects. Simultaneously, improvements in gene delivery technologies are required to enhance the effective delivery and persistence of gene therapy. Additionally, a deeper understanding of genetic diseases, including disease mechanisms, pathogenic genes, and key factors in disease progression, is essential. This will contribute to better identifying treatment targets and selecting appropriate therapeutic strategies.
In the realm of clinical trials and regulation, it is imperative to conduct more clinical trials to assess the efficacy and safety of gene editing and gene therapy. Simultaneously, the establishment of robust regulatory frameworks is essential to ensure the rationality and feasibility of treatments. Leveraging individual genomic information to achieve personalized treatment for genetic diseases is crucial. By integrating gene editing and gene therapy, tailored treatment plans can be devised for each patient. Strengthening collaboration among scientists, clinicians, ethicists, and legal experts is necessary, Considering fully consideration ethical, legal, and societal issues, and formulating appropriate policies and guidelines.
In conclusion, gene editing and gene therapy hold significant promise in the treatment of genetic diseases. With ongoing technological advancements and deeper clinical research, gene editing and gene therapy are poised to play even more substantial roles in the future of treating genetic diseases, further enhancing the quality of life and health outcomes for patients.
Acknowledgments
Special thanks to Mrs. Xuan Jia and Mrs. Zhang Jie for their guidance and review of the paper. Many of their constructive comments have greatly contributed to the improvement of this research.
Hu S.H., Liu Q.Y., Xie D.C., and Huang J.J., 2022, Clinical research progress of CRISPR/Cas genome editing in the treatment of human genetic disorders, Shengming Kexue (Chinese Bulletin of Life Sciences), 34(10): 1250-1263.
Liao Y., Zeng X.L., and Yang J., 2022, Application progress of fmr1 gene in diagnosis and treatment of fragile x syndrome, medical recapitulate, (12): 2476-2481.
https://doi.org/10.12677/ACM.2022.12121710
Li C.H., Hu B., Weng Y.H., Huang Y.Y., 2019, current situation and clinical research progresses of gene therapy,Shengming Kexue Yiqi (Life Science Instruments), 17(4): 3-12.
Li X., Chui W.T., and Li K., Research advances on techniques of gene editing and its application, Zhongguo Xumu Shouyi (China Animal Husbandry & Veterinary Medicine), 44(8): 2241-2247.
Wang H.Y., Li P.F., Xu L.J., Zhang L.W., He C.H., Fan Y.L., Yu J.R., Xu Z.H., 2021, Ethical governance of gene editing technology, Zhonguo Kexueyuan Yuankan (Bulletin of Chinese Academy of Sciences), 36(11): 1259-1269.
Yang X.Y., Wang D.Y., and Gao X., 2020, The Application of gene editing technology and cell therapy in in vitro gene therapy, Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao (Chinese Journal of Biochemistry and Molecular Biology), 36(11): 1265-1272.
Zhang F., Zong Y., MaW., Xu D., Wu J.Y., Construction of congenital heart disease key gene expression vector by Crispr/Cas9 technology, Lanzhou Daxue Xuebao (Journal of Lanzhou University (Medical Sciences)), 46(1): 72-76.
Zhou L.J., Wang J.C., Gao H.B., Zhao D.P., Gene therapy for monogenetic diseases, Keji Daobao (Science & Technology Review), 38(15): 89-100.
Zhu B.y., Li L.X., Wang L.R., and Li D.l., 2019, Recent ex vivo gene therapy through genome editing, Zhonguo Xibao Shengwuxue Xuebao (Chinese Journal of Cell Biology), (4): 573-582.
. PDF(232KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Xiaoping Cai
Related articles
. Gene editing
. Gene therapy
. Genetic diseases
. CRISPR-Cas9
. Gene correction
Tools
. Email to a friend
. Post a comment