Research Article
Application and Prospects of Gene Editing Technology in the Treatment of Neurological Disorders 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 1 doi: 10.5376/ijmms.2024.14.0006
Received: 16 Feb., 2024 Accepted: 18 Mar., 2024 Published: 29 Mar., 2024
Wu J., 2024, Application and prospects of gene editing technology in the treatment of neurological disorders, International Journal of Molecular Medical Science, 14(1): 42-47 (doi: 10.5376/ijmms.2024.14.0006)
Neurological diseases are a serious threat to human health, causing a huge burden on patients and society. Traditional treatment methods often cannot effectively cure neurological diseases, so it is necessary to explore new treatment strategies. The rapid development of gene editing technology has provided new ideas for the treatment of neurological diseases. This review will systematically review the application of gene editing technology in the treatment of neurological diseases. The review provides an overview of the principles and tools of gene editing technology, analyzes the characteristics of neurological diseases and the challenges of existing treatments, and explores in detail the application cases of gene editing technology in the treatment of neurological diseases such as neurodegenerative diseases and congenital neurological diseases. In addition, this review also discusses the challenges and safety considerations faced by gene editing technology, and looks forward to the future prospects and development directions. Comprehensive analysis shows that gene editing technology has enormous advantages and potential in the treatment of neurological diseases, bringing new hope to people.
Neurological disorders are a type of disease that seriously affects human health and quality of life, including neurodegenerative diseases and congenital neurological disorders. These diseases bring enormous psychological, economic, and social burdens to patients and their families, while also posing significant challenges to the entire healthcare system. Traditional treatment methods often only alleviate symptoms and cannot cure diseases, so there is an urgent need to find more effective treatment methods.
The rise of gene editing technology has brought hope for the treatment of neurological diseases. As a powerful tool, gene editing technology has shown great potential in changing the treatment methods of neurological diseases. Gene editing technology can achieve precise disease treatment by directly modifying abnormal genes in the genome, repairing damaged genes, or enhancing gene expression. The CRISPR-Cas9 system, which has recently attracted widespread attention, has made significant breakthroughs in the field of gene therapy as an efficient, precise, and programmable gene editing tool (Bhattacharjee, 2022).
By precisely modifying the genetic material within cells, gene editing technology provides a new approach to treat the underlying genetic causes of various neurological diseases. This groundbreaking technology has great potential for treating congenital and genetic neurological diseases such as Huntington's disease, amyotrophic lateral sclerosis, and certain types of epilepsy. In addition, gene editing technology can also be used to manipulate genes related to neurodegenerative diseases, neuroinflammation, and even malignant brain tumors (Gumusgoz et al., 2021). As the field continues to progress and research deepens, gene editing technology has opened up new avenues for personalized medicine in the treatment of neurological diseases, bringing new hope and better treatment outcomes to patients.
This review systematically reviews the application of gene editing technology in the treatment of neurological diseases and looks forward to its prospects. The paper introduces the principle of gene editing technology, discusses in detail the application of gene editing technology in the treatment of neurodegenerative diseases, explores the potential of gene editing technology in the treatment of congenital neurological diseases, and looks forward to the application prospects of gene editing technology in the treatment of neurological diseases. Although gene editing technology has enormous potential and advantages, it also faces many challenges, such as technical limitations and safety issues. This review promotes understanding and prospects for the application of gene editing technology in the treatment of neurological diseases. We hope that through this review, we can further promote the research and clinical application of gene editing technology, and provide more effective treatment plans for patients with neurological diseases.
1 Overview of Gene Editing and Neurological Diseases
1.1 Basic principles of gene editing
Gene editing technology is a technique that can directly modify the genome of organisms and is widely used in disease research and treatment. In neurological diseases, gene editing technology has enormous potential to accurately locate and repair disease-related gene mutations, thereby changing the pathogenesis and progression of the disease, and bringing new treatment options to patients.
Gene editing technology is mainly based on the CRISPR-Cas9 system, which is a natural defense mechanism derived from the bacterial immune system. The CRISPR-Cas9 system is composed of Cas9 protein and a directed RNA segment (sgRNA) (Bhattacharjee, 2022). Cas9 is an endonuclease used to cleave target DNA sequences, while sgRNA is an RNA molecule used to guide Cas9 protein to the target DNA sequence.
The process of gene editing includes three main steps. Design and construct sgRNAs, by calculating the complementarity between target sequences in the genome and sgRNAs, suitable sgRNAs can be designed. Cas9 mediated target DNA cleavage, Cas9 protein and sgRNA form complexes, recognize and bind to specific positions in the target DNA sequence, and cleave the target DNA, resulting in double stranded breaks (Figure 1). DNA repair and genetic modification, cells use repair pathways such as non homologous end connections (NHEJ) and homologous recombination (HDR) to repair Cas9 induced DNA breaks. The NHEJ repair process may lead to insertion or deletion mutations, while the HDR repair process provides an opportunity for precise insertion, deletion, or repair of mutations.
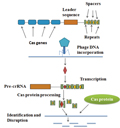 Figure 1 Cas protein mediated DNA disruption and identification |
Gene editing technology has the advantages of high efficiency, speed, and precise control of target gene modifications. It can not only be used to repair mutations, but also to knock out or insert genes, thereby changing the function and expression level of genes. Therefore, gene editing technology has been widely applied in the construction of disease models, gene function research, and treatment and prevention of diseases.
1.2 Classification and characteristics of common neurological diseases
Neurological diseases can be classified differently based on their characteristics. Neurodegenerative diseases are a common type of disease characterized by progressive degeneration or damage to the structure and function of the nervous system. These diseases are usually caused by genetic factors, which can lead to the death, loss of function, or degeneration of nerve cells, thereby affecting the normal functioning of the nervous system. Alzheimer's disease, Parkinson's disease, and Huntington's disease are several typical examples of neurodegenerative diseases.
On the other hand, congenital neurological disorders refer to neurological abnormalities or defects that exist during embryonic development or birth. These diseases can be caused by genetic factors, environmental factors, or their interaction. Spinal meningocele, cerebellar hypoplasia, and phenylketonuria are several common examples of congenital neurological disorders (Li et al., 2022). These diseases have a significant impact on the lives and functions of patients. Neurodegenerative diseases and congenital nervous system diseases differ in their characteristics and pathogenesis, but their commonality is that they cause varying degrees of damage to the structure and function of the patient's nervous system, requiring attention to research and treatment efforts.
The research and treatment of neurological diseases are of great significance. Early diagnosis and intervention are crucial for the prognosis and quality of life of patients. In recent years, with the development of gene editing technology, new opportunities and hope have been provided for the research and treatment of these diseases. Gene editing technology can help understand the pathogenesis of these diseases, develop personalized treatment plans, and provide new avenues for correcting neurological defects, such as locating and repairing mutations in related genes, or through gene therapy and other means. These advances bring hope for improving the prognosis and quality of life of patients.
2 The Application of Gene Editing Technology in the Treatment of Neurological Diseases
2.1 Application in the treatment of neurodegenerative diseases
Gene editing technology has broad application prospects in the treatment of neurodegenerative diseases (Ekman et al., 2019). These technologies can be used to study the pathogenesis of diseases, develop personalized treatment strategies, and provide patients with new treatment options. Among them, CRISPR-Cas9 is a revolutionary gene editing technique that can be used to precisely modify the genome, such as knocking out or modifying specific genes. In neurodegenerative diseases, CRISPR-Cas9 can be used to evaluate gene function and repair genes with pathogenic mutations (Amin et al., 2018).
Gene therapy is also a method of applying gene editing technology, which can restore the function of damaged nerve cells by introducing normal genes into the patient's cells. Gene editing technology can also be used to enhance the immune system's treatment of neurodegenerative diseases, such as by editing T cells to more effectively recognize and destroy harmful protein deposits. In addition, gene editing technology can also be used to create animal models (Figure 2), study the development process of human neurodegenerative diseases, and evaluate new treatment strategies (Ma et al., 2020).
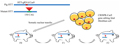 Figure 2 HD (Huntington's disease) gene edited (knocked in) pig model established using CRISPR-Cas9 technology |
Although gene editing technology has shown potential in the treatment of neurodegenerative diseases, challenges and ethical issues still need to be faced. Due to their safety and effectiveness, the research and application of these technologies require prospective clinical trials and regulation. In addition, it is necessary to weigh potential risks and benefits to protect the interests of patients and the public. In summary, gene editing technology has important application prospects in the treatment of neurodegenerative diseases, and its development provides new hope for improving the prognosis and quality of life of patients.
2.2 Application in the treatment of congenital neurological diseases
Gene editing technology has important application potential in the treatment of congenital neurological diseases. These diseases refer to neurological developmental or genetic abnormalities that occur during fetal or early childhood. Gene editing technology can be used to repair or restore the function of abnormal genes, knock out harmful genes, or introduce missing genes. By using tools such as CRISPR-Cas9, precise editing of problematic genes can be achieved. In addition, gene editing technology can also be used to create animal models that simulate specific gene mutations or injuries in human congenital nervous system diseases (Zhu et al., 2023). This model can help study the development mechanisms of diseases and evaluate the effectiveness of new treatment strategies.
Although gene editing technology has great potential in the treatment of congenital neurological diseases, further research and clinical practice are still needed. Safety, effectiveness, and ethical issues all need to be carefully considered and fully evaluated. However, the application of these technologies is of great significance for finding new treatment methods and improving the quality of life of patients with congenital neurological diseases.
2.3 Application in the treatment of other neurological diseases
Gene editing technology also has potential in the treatment of other neurological diseases besides neurodegenerative diseases and congenital neurological diseases. This includes neuromuscular diseases, neuralgia, spontaneous motor disorders, and cerebrovascular diseases. For neuromuscular diseases, gene editing can restore normal nerve and muscle function and alleviate disease symptoms by repairing related gene mutations. In the treatment of neuralgia, gene editing technology can regulate genes related to pain transmission and alleviate symptoms of neuralgia.
For spontaneous motor disorders, gene editing technology can improve motor function and reduce the onset of symptoms by repairing related gene mutations. For cerebrovascular diseases, gene editing technology can enhance the regeneration and repair process of damaged nerve cells, promote vascular growth, and restore brain tissue. Although more research is still needed for the application in these fields, gene editing technology provides promising progress in the treatment of other neurological diseases, with potential positive impacts on patient recovery and quality of life.
3 The Prospect of Gene editing Technology in the Treatment of Neurological Diseases
3.1 Advantages of gene editing in the treatment of neurological diseases
Gene editing technology has many advantages in the treatment of neurological diseases. Gene editing technology has high precision, which can accurately repair or modify target genes, minimize the impact on other genes, improve treatment effectiveness, and reduce risk (Song et al., 2020). Gene editing technology has therapeutic potential, which can repair harmful gene mutations related to diseases, restore or enhance the normal function of nerve cells, improve disease symptoms, and reduce disease progression.
In addition, the long-lasting effect of gene editing technology is also one of its advantages. Through one-time gene editing therapy, it can continuously produce therapeutic effects, avoiding the need for long-term drug treatment. Individualized treatment is another important advantage, and gene editing technology can provide customized treatment based on the patient's genotype and condition. Gene editing technology has diverse applications in the treatment of neurological diseases, including repairing gene mutations, inhibiting the expression of disease-related genes, and increasing the recovery ability of nerve cells.
However, it should be pointed out that gene editing technology is still in its early stages and faces some challenges, such as ensuring safety, improving delivery methods, and addressing ethical and moral issues. Therefore, further research and clinical practice are necessary to determine the feasibility and reliability of its widespread application in the treatment of neurological diseases.
3.2 Technical limitations and security issues
Although gene editing technology has great potential in the treatment of neurological diseases, it also faces some technical limitations and safety issues. The delivery challenge is one of them, and delivering editing tools to neural cells is a challenge in gene editing technology. The current delivery methods may be limited, including low delivery efficiency and difficulty in penetrating the blood-brain barrier. Developing more effective delivery systems is crucial for achieving successful treatment of neurological diseases. The issue of cell type specificity also needs to be considered. The nervous system is very complex, including various types of cells with different functions and characteristics. Gene editing technology needs to ensure that editing is only performed on target cells to avoid unexpected effects on other cell types.
Although gene editing technology may be very useful in treatment, it also has safety issues. For example, unexpected splicing or addition events may trigger gene mutations unrelated to the disease, and even lead to other health issues. Therefore, ensuring the safety of gene editing technology is very important. In addition, when using gene editing technology to treat neurological diseases, ethical and moral issues also need to be considered (Shi et al., 2021). For example, for certain genetic diseases, gene editing may be necessary in the early stages of embryonic development, which raises a series of ethical and social value related issues.
3.3 Future development direction
The future development direction of gene editing technology in the treatment of neurological diseases is very broad. Improving delivery systems is a key direction, and in response to the delivery challenges of current gene editing technology, researchers will continue to improve the efficiency and penetration of delivery systems to enhance the ability of editing tools to reach nerve cells. Possible methods include nanoparticles, viral vectors, etc. Further improvement is needed in the accuracy and specificity of gene editing techniques to ensure that editing is only performed in target cells and to minimize the impact on other cells. Researchers can explore new editing tools, editing techniques, and achieve their goals by improving Cas9 or developing more precise editing tools.
Combination therapy strategies are also a promising direction, such as drug therapy, photogenetics, and electrical stimulation, to enhance treatment effectiveness. By comprehensively applying multiple treatment methods, interventions can be targeted at different aspects of the disease to improve the success rate of treatment (He et al., 2021). In addition, the development of genomics is crucial for understanding neurological diseases and developing new editing targets. By studying the genomic information of patients with neurological diseases, new targets and mutations related to the disease can be discovered, and gene editing techniques can be developed and applied for repair. The development of genomics will enhance the understanding of neurological diseases and provide better basis for personalized treatment. Before further development of gene editing technology in the treatment of neurological diseases, it is necessary to establish stricter clinical trials and regulatory frameworks to ensure the safety and effectiveness of treatment, and to address ethical and legal issues.
Overall, the future development directions of gene editing technology in the treatment of neurological diseases include improving delivery systems, increasing the accuracy of editing techniques, combining treatment strategies, genomics research, and strengthening clinical applications and regulation. These developments will further promote the application and effectiveness of gene editing technology in the treatment of neurological diseases.
4 Summary and Outlook
Gene editing technology, as an advanced biomedical tool, has shown great potential for application in the treatment of neurological diseases. Gene editing technology has been widely applied in cutting-edge research and clinical trials of neurological diseases, achieving some encouraging results. By precisely editing and repairing mutations in relevant genes, gene editing technology provides a new therapeutic approach for the treatment of congenital neurological diseases. In addition, gene editing can also be used to inhibit the occurrence of neurological tumors and inflammatory responses (Pazzaglia and Pioli, 2019), providing new strategies for the treatment of neurodegenerative diseases.
However, despite the enormous potential of gene editing technology in the treatment of neurological diseases, it still faces challenges in delivery efficiency, accuracy, safety, and ethics. It is necessary to address the limitations of gene editing technology in order to achieve its wider application in the treatment of neurological diseases. The key to future development is to improve the efficiency and penetration of delivery systems, enhance the accuracy of editing techniques, and establish strict clinical trial and regulatory frameworks. Through the comprehensive application of various treatment strategies, such as combination therapy, gene editing technology is expected to bring better results to the treatment of neurological diseases. In addition, advances in genomics will provide a better foundation for precise treatment of neurological diseases.
In summary, gene editing technology has shown great potential in the treatment of neurological diseases, and future research and development will continue to promote progress in this field. Improving delivery systems, increasing the accuracy of editing techniques, combining treatment strategies, genomics research, and strengthening clinical applications and supervision will promote the further development of gene editing technology in the treatment of neurological diseases, bringing better therapeutic effects to patients.
Amin M., Shahid K., Nasir R., Alam I., and Anwar P., 2018, CRISPR/Cas9: A revolution towards, treatment for neurodegenerative disorders, Open J Biotechnol Bioeng Res. 2(1): 001-008.
Bhattacharjee G., Gohil N., Khambhati K., Mani I., Maurya R., Karapurkar J.K., Gohil., Chu D.T., Vu-Thi H., Alzahrani K.J., Show P.L., Rawal R.M., Ramakrishna S., and Singh V., 2022, Current approaches in CRISPR-Cas9 mediated gene editing for biomedical and therapeutic applications, Journal of Controlled Release, 343: 703-723.
https://doi.org/10.1016/j.jconrel.2022.02.005
PMid:35149141
Ekman F.K., Ojala D.S., Adil M.M., Lopez P.A., Schaffer D.V., and Gaj T., 2019, CRISPR-Cas9-mediated genome editing increases lifespan and improves motor deficits in a huntington’s disease mouse model, Molecular Therapy Nucleic Acids, 17: 829-839.
https://doi.org/10.1016/j.omtn.2019.07.009
PMid:31465962 PMCid:PMC6717077
Gumusgoz E., Guisso D.R., Kasiri S., Wu J., Dear M., Verhalen B., Nitschke S., Mitra S., Nitschke F., and Minassian B.A., 2021, Targeting Gys1 with AAV‐SaCas9 decreases pathogenic polyglucosan bodies and neuroinflammation in Adult Polyglucosan Body and Lafora disease mouse models, Neurotherapeutics, 18: 1414-1425.
https://doi.org/10.1007/s13311-021-01040-7
PMid:33830476 PMCid:PMC8423949
He C.H., Jiang W.Z., Zhang L.W., Ruan M.H., Zhou H.W., and Yu J.R., 2021, Current status and future perspectives of rare disease research, Yichuan (Hereditas(Beijing)), 43(6): 531-544.
Hitti F.L., Gonzalez-Alegre P., and Lucas T.H., 2019, Gene therapy for neurologic disease: a neurosurgical review, World Neurosurgery, 121: 261-273.
https://doi.org/10.1016/j.wneu.2018.09.097
PMid:30253990
Li X.S., Wang Q.S., and Wang R., 2022, Advantages of CRISPR-Cas9 combined organoid model in the study of congenital nervous system malformations, Front. Bioeng. Biotechnol, 10: 932936.
https://doi.org/10.3389/fbioe.2022.932936
PMid:36118578 PMCid:PMC9478582
Ma B.X., Shen W.L., Wang X., Li Z., and Xu K., 2020, Gene edited animal models applied in human disease research, Shengwu Gongcheng Xuebao (Chinese Journal of Biotechnology), 36(5): 849-860.
Pazzaglia S., and Pioli C., 2019, Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases, Cells, 9(1): 41.
https://doi.org/10.3390/cells9010041
PMid:31877876 PMCid:PMC7017201
Shi M.G., Shen Z.Y., Zhang N., Wang L.Y., Yu C.Y., and Yang Z., 2021, CRISPR/Cas9 technology in disease research and therapy: a review. Chinese Journal of Biotechnology, 37(4): 1205-1228.
Song S.Z., Lu R., Zhang T., He Z.Y., Wu Z.M.Q., Cheng Y., and Zhou M.M., 2020, Research progress of CRISPR /Cas9 gene editing technology in goat and sheep, Biotechnology Bulletin, 36(3): 62-68.
Zhu X.C, Xie X.Y., Zhao X.R., Xu L.N., He Z.Y., and Zhou W., 2023, Construction and characterization of mice with conditional knockout of Stat3 gene in microglia, Shanghai Jiaotong Daxue Xuebao (Journal of Shanghai Jiao Tong University (Medical Science)), 43(6): 689-698.
. PDF(238KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Jenny Wu
Related articles
. Gene editing
. Neurological disorders
. CRISPR-Cas9
. Neurodegenerative diseases
. Congenital neurological disorders
Tools
. Email to a friend
. Post a comment