Research Article
The Application and Challenges of Mesenchymal Stem Cells' Immunomodulation in the Treatment of Autoimmune Diseases 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 2
Received: 04 Apr., 2024 Accepted: 07 May, 2024 Published: 15 May, 2024
Mesenchymal stem cells (MSCs) have become a research focus in the treatment of autoimmune diseases due to their unique immunomodulatory function and multidirectional differentiation potential. MSCs can effectively regulate inflammatory response and immune balance by secreting anti-inflammatory and immune regulatory factors and directly interacting with immune cells. This study reviews the application, challenges, and future research directions of MSCs in the treatment of autoimmune diseases. Studies have shown that MSCs have shown potential therapeutic efficacy in clinical trials for diseases such as systemic lupus erythematosus, rheumatoid arthritis and Crohn's disease, reducing symptoms and improving patients' quality of life. However, its clinical application still faces dual challenges of safety and efficacy, including inconsistencies in cell source and preparation process, long-term survival and transformation risks. Future research should focus on optimizing the production and application processes of MSCs, exploring ways to enhance their immunomodulatory effects through genetic engineering and bioengineering techniques, and verifying their safety and efficacy through large-scale clinical trials. Through continued research and technological innovation, MSCs are expected to provide an effective new strategy for the treatment of autoimmune diseases.
Autoimmune diseases are diseases in which the immune system attacks the body's own tissues due to disorders, including but not limited to systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis and so on. Autoimmune diseases not only cause long-term pain and dysfunction in patients, but also may lead to multiple organ damage, seriously affecting patients' quality of life and life expectancy (Huang et al., 2022).
In the field of the treatment of autoimmune diseases, traditional treatments have mainly relied on the use of drugs such as immunosuppressants and glucocorticoids to control the condition. However, these treatments are often associated with long-term side effects and limited therapeutic effectiveness. Therefore, the development of new treatment strategies is particularly important. In recent years, mesenchymal stem cells (MSCs) have emerged as a novel therapeutic strategy for treating autoimmune diseases due to their unique immunomodulatory properties (Ben-Ami et al., 2011).
MSCs are a class of non-hematopoietic stem cells with multidirectional differentiation potential, which can be extracted from bone marrow, adipose tissue, cord blood and other tissues. They can regulate the immune response by secreting a variety of immunomodulatory molecules such as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), thereby combating inflammation and immune overreaction. In addition, MSCs further exert their immunomodulatory role by interacting with a variety of cells of the immune system such as T cells, B cells, and macrophages, which provides a scientific basis for their application in the treatment of autoimmune diseases (Gomzikova et al., 2019).
Studying the use of MSCs in autoimmune diseases could not only provide a potential treatment for these diseases, but could also help address deficiencies in traditional treatment options. Exploring the immunomodulatory mechanism of MSCs and its clinical application potential is of great significance for promoting scientific research and clinical treatment in this field.
1 Immunomodulatory Properties of Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are highly versatile non-hematopoietic stem cells that can be isolated from a variety of adult tissues, including bone marrow, adipose tissue, dental pulp, cord blood, etc. These cells have the ability to self-renew and differentiate into a variety of cell types, such as osteoblasts, fat cells, and chondrocytes. In addition to their regenerative capacity, MSCs also exhibit significant immunomodulatory functions, which makes them show great potential in the treatment of a variety of diseases, especially autoimmune diseases.
1.1 Immune regulatory mechanism of MSCs
1.1.1 Direct mechanism
Chen et al. (2019) mentioned in their study that the direct mechanism involves physical contact between MSCs and immune cells such as T cells, B cells, natural killer cells, and dendritic cells. MSCs interact with immune cells by expressing a range of surface molecules that affect the activity of these cells. For example, MSCs can bind to PD-1 on T cells by expressing programmed death ligand-1 (PD-L1), thereby inhibiting T cell activation and proliferation. In addition, MSCs can also affect B cell maturation and antibody production through direct intercellular contact.
1.1.2 Indirect mechanism
Gao et al. (2016) proposed that the indirect mechanism affects the function of immune cells through a variety of solute factors secreted by MSCs, such as cytokines and chemical factors. MSCs are able to secrete a variety of immunomodulatory molecules, such as transforming growth factor-β (TGF-β), interleukin-10 (IL-10), and interleukin-6 (IL-6), which can regulate immune responses in the absence of direct cellular contact. For example, TGF-β and IL-10 are potent anti-inflammatory cytokines that inhibit the activation of inflammatory cells and promote immune tolerance. MSCs further inhibit the function of immune cells by secreting molecules such as inducible nitric oxide synthase (iNOS) and prostaglandin E2 (PGE2).
1.2 Molecular mechanisms and signaling pathways of MSCs in immune regulation
Huang et al. (2022) mentioned that the immunomodulatory function of MSCs involves a variety of cell signaling pathways, and the activation or inhibition of these pathways affects the behavior of immune cells. Studies have shown that MSCs communicate with immune cells through the Notch signaling pathway and regulate the differentiation and function of immune cells. MSCs are also involved in immune regulation through the Wnt/β-catenin signaling pathway, which plays a key role in regulating inflammatory response and immune tolerance.
Gomzikova et al. (2019) proposed that MSCs modulate immune responses through STAT3 and NF-κB signaling pathways. Activation of these signaling pathways can promote the secretion of anti-inflammatory cytokines by MSCs and inhibit the activation of immune cells and inflammatory response. Through these complex molecular mechanisms, MSCs are able to play a regulatory role in the immune system, providing a new therapeutic strategy for the treatment of autoimmune diseases. Through their unique immunomodulatory function, mesenchymal stem cells have shown great potential in the treatment of autoimmune diseases. The play of these functions depends on the interaction of multiple complex molecular mechanisms and signaling pathways, which need to be further explored and verified in future studies.
2 Application of Mesenchymal Stem Cells in the Treatment of Autoimmune Diseases
Mesenchymal stem cells (MSCs) have been widely studied for the treatment of various autoimmune diseases due to their excellent immunomodulatory properties. These diseases often result from chronic inflammation and tissue damage caused by the immune system's attack on their own tissues, and MSCs are able to intervene in these pathological processes to improve the condition by regulating the immune response and promoting tissue repair. The following sections will discuss in detail the application of MSCs in the treatment of autoimmune diseases and related research progress.
2.1 Clinical cases and research progress of MSCs in the treatment of autoimmune diseases
With the continuous progress of medicine, mesenchymal stem cells (MSCs), as a promising therapeutic method, have achieved remarkable results in the treatment of autoimmune diseases. This unique cell type has become a research hotspot in the field of autoimmune disease therapy due to its multi-potential, low immunogenicity and immunomodulatory properties.
Yi and Song (2012) mentioned in their study that the therapeutic potential of MSCs has been preliminarily verified in in vitro experiments and animal models. Subsequently, a series of clinical trials further confirmed the efficacy of MSCs in patients with autoimmune diseases. For example, systemic lupus erythematosus (SLE) is a typical autoimmune disease characterized by the immune system mistakenly attacking its own healthy cells and tissues. Intravenous administration of MSCs can significantly improve the symptoms and biochemical markers of SLE patients. These improvements included reduced levels of anti-double-stranded DNA antibodies, increased complement levels, and increased survival for patients. This study provides strong support for the application of MSCs in the treatment of SLE (Figure 1).
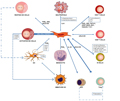 Figure 1 Potential mechanisms of the MSCs interactions with immune cells (Yi and Song, 2012) |
According to the study of Kong et al. (2009), in addition to SLE, MSCs have also shown good efficacy in the treatment of rheumatoid arthritis (RA). RA is a chronic autoimmune disease, mainly manifested by joint inflammation, pain, stiffness and other symptoms. By local injection or intravenous injection of MSCs, it has been found that joint inflammation, pain and morning stiffness symptoms in RA patients can be significantly reduced, and joint function can be improved. These positive therapeutic effects are partly attributed to the anti-inflammatory effects of MSCs and their regulation of immune cell function. MSCs can inhibit inflammation and promote tissue repair by secreting a variety of bioactive molecules, such as growth factors and anti-inflammatory factors.
2.2 Application of immune regulation of mesenchymal stem cells in specific autoimmune diseases
MSCs have significant potential and advantages in the treatment of autoimmune diseases. They not only directly inhibit the abnormal activity of the immune system, but also promote tissue repair and regeneration, providing a new direction for the treatment of these diseases. Future studies are needed to further optimize treatment regimens for MSCs, such as determining the most effective cell dose, timing and route of administration, as well as evaluating long-term safety and efficacy, to better translate this therapeutic strategy into clinical applications.
2.2.1 Systemic Lupus Erythematosus (SLE)
Autoimmune diseases are a class of diseases caused by the body's immune system mistakenly attacking its own healthy cells and tissues. Systemic lupus erythematosus (SLE) is a complex multi-system autoimmune disease with symptoms involving multiple organs and systems, including the skin, joints, kidneys, heart, and nervous system. The treatment of SLE has always been a challenge, mainly because of the need to control an overactive immune response while preventing tissue damage. In recent years, the application of mesenchymal stem cells (MSCs) in the treatment of autoimmune diseases has attracted wide attention, especially in the treatment of SLE.
Chen et al. (2019) believed that in the treatment of SLE, MSCs secrete anti-inflammatory cytokines such as TGF-β and IL-10 to suppress the excessive immune response, thereby alleviating the symptoms of SLE patients. In addition, MSCs are able to restore balance to the immune system by promoting the function of regulatory T cells (Tregs). Tregs are a class of immunosuppressive T cells that suppress the autoimmune response, thereby protecting the body from autoimmune diseases. Studies have shown that MSCs can induce the production of Tregs and enhance their immunosuppressive function, thereby further suppressing the autoimmune response and alleviating the symptoms of SLE patients. In addition to acting directly on the immune system.
Gomzikova et al. (2019) believe that although MSCs show some potential in the treatment of SLE, the current research is still in the preliminary stage. In the future, it is necessary to further investigate the mechanism of action and therapeutic effect of MSCs, and explore its application in other autoimmune diseases. At the same time, attention should also be paid to the safety and feasibility of MSCs treatment to ensure that they can be safely and effectively used in clinical practice.
2.2.2 Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by joint inflammation, pain, swelling, and joint destruction. For the treatment of RA, the key is to effectively control joint inflammation, prevent joint destruction, and restore joint function. In the treatment of RA, the application of MSCs is mainly based on their anti-inflammatory, immunomodulatory, and tissue repair capabilities. MSCs can inhibit inflammatory cell infiltration and the production of inflammatory factors by secreting a variety of anti-inflammatory factors, such as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10). These anti-inflammatory factors can down-regulate the expression of pro-inflammatory cytokines, reduce the inflammatory response in the joint cavity, and thus relieve joint pain and swelling.
Munir and McGettrick (2015) mentioned that MSCs also have immunomodulatory functions, which can regulate the activity of immune cells and inhibit autoimmune reactions. In the pathogenesis of RA, immune cells such as T cells, B cells and macrophages are involved in the occurrence and development of joint inflammation. By interacting with immune cells, MSCs can inhibit their activation, proliferation and differentiation, thereby reducing the progression of joint inflammation. MSCs also promote the regeneration and repair of articular cartilage. In the course of RA, the destruction of articular cartilage is one of the important reasons leading to the loss of joint function. MSCs can differentiate into chondrocytes, secrete cartilage matrix, and promote the regeneration and repair of joint cartilage. Through this mechanism, MSCs can slow down the process of joint destruction and protect joint function. Through in-depth study of the biological characteristics and mechanism of MSCs, it is expected to provide new strategies and methods for the treatment of RA.
2.2.3 Crohn's disease
Crohn's disease, a chronic inflammatory bowel disease, has become the focus of medical attention because of its difficult to cure. Patients often suffer from diarrhea, abdominal pain, weight loss and other symptoms, and their quality of life is severely affected. MSCs are a class of cells with self-renewal ability and multidirectional differentiation potential, which can regulate immune response and promote tissue repair. In the treatment of Crohn's disease, the potential of MSCs is mainly reflected in two aspects: First, by regulating intestinal immune response, inhibiting excessive inflammatory response, thereby reducing intestinal tissue damage; The second is to promote the repair of damaged intestinal mucosa, accelerate the regeneration of intestinal epithelial cells, and restore intestinal function.
Dalal et al. (2012) mentioned that in order to give full play to the therapeutic effect of MSCs, researchers adopted the method of local intestinal application. By injecting MSCs directly into a patient's gut, they can be made to work better. Experiments have shown that the local application of MSCs can significantly reduce the infiltration of inflammatory cells, reduce the degree of intestinal inflammation, and promote the healing of intestinal epithelial cells and accelerate the repair process of intestinal mucosa. In addition, MSCs also have powerful immunomodulatory effects. They can secrete a variety of bioactive molecules, such as anti-inflammatory factors, growth factors, etc., which can effectively regulate the activity of intestinal immune cells and reduce the damage of immune response to intestinal tissue. By modulating the immune response, MSCs create a more conducive environment for the gut to recover.
Ringden et al. (2022) mentioned that clinical trials of MSCs in the treatment of Crohn's disease have achieved certain results. Many patients experience significant improvement in symptoms and quality of life after receiving MSCs treatment. However, due to individual differences and the complexity of the disease, the effectiveness of MSCs treatment is not satisfactory. Therefore, how to further improve the efficacy and safety of MSCs treatment is still a topic that scientists need to further study (Figure 2).
.jpg) Figure 2 Immunomodulatory and regenerative properties of MSCs (Ringdén et al., 2022) |
3 Challenges and Problems Faced
3.1 Challenges in the clinical application of MSCs
3.1.1 Security
Munir and McGettrick (2015) believe that although MSCs have shown relatively good safety in clinical trials, there are still some safety hazards that need to be paid attention to. First, the diversity of sources and the different preparation processes of MSCs may lead to inconsistencies in their biological activities, which may affect the safety and efficacy of clinical applications. Second, the long-term survival and potential transformation risk of MSCs in vivo remains an unresolved question. For example, although rare, studies have reported the risk of tumor formation that MSCs may cause in vivo .
3.1.2 Validity
Chen et al. (2019) argued that the efficacy of MSCs showed significant variability across different clinical trials, which could be caused by a variety of factors, such as cell dose, route of administration, patient disease status, and background. In addition, the mechanism of action of MSCs is complex, and their immunomodulatory function is closely related to the patient's own immune environment, which increases the difficulty of predicting their clinical effects.
3.2 Complexity and unpredictability of immune regulatory mechanisms
Castro-Manrreza and Montesinos (2015) mentioned that the immunomodulatory functions of MSCs involve multiple cell types and complex signaling pathways, which makes their immunomodulatory mechanisms extremely complex. Although some of the mechanisms by which MSCs interact with immune cells have been revealed, there are still a lot of unknown areas to explore. This complexity leads to variability and unpredictability in the response of MSCs to individuals in practical applications, which increases the risk and uncertainty of treatment.
3.3 Evaluation of safety and efficacy of cell therapy
Peng et al. (2020) believe that when MSCs are used to treat autoimmune diseases, their safety and efficacy must be rigorously evaluated. This includes aspects such as the source, quality, quantity, purity of the cells and the long-term effect after treatment. At present, the lack of a unified and standardized evaluation system makes the evaluation of the safety and efficacy of MSCs treatment challenging.
3.4 Technical challenges of cell origin, isolation and culture
Sources of MSCs are limited and are usually obtained from tissues such as bone marrow, fat, and cord blood. This limits its wide application in clinical practice. In addition, the isolation and culture of cells is also a technical challenge. Currently, while there are ways to isolate and grow MSCs from these tissues, these methods are often time-consuming, inefficient, and costly.
3.5 Legal, ethical and cost considerations
As Ringden et al. (2022) mentioned in their study, MSCs treatment involves multiple aspects such as ethics, law and economy. For example, the legality and compliance of cell sources, the right to information and privacy of patients, and the affordability of treatment costs all need to be considered. These factors may limit the widespread clinical use of MSCs therapy. Therefore, it is necessary to fully consider these aspects when formulating relevant policies and regulations to promote the healthy development of MSCs treatment.
4 Prospect and Research Direction
4.1 Application prospect of MSCs in the treatment of autoimmune diseases
Chen et al. (2019) proposed in their study that MSCs have the potential to regulate inflammation, promote tissue repair and maintain immune homeostasis, making them an ideal choice for the treatment of autoimmune diseases. These cells can secrete a variety of anti-inflammatory cytokines and other solute factors, such as transforming growth factor-β (TGF-β), interleukin-10 (IL-10), and so on, effectively inhibit the inflammatory response. At the same time, they can also engage in complex interactions with immune cells, such as T cells and B cells, to regulate the activity of the immune system and prevent excessive immune responses from causing tissue damage.
Take systemic lupus erythematosus, a disease caused by the immune system's abnormal attack on its own tissues. Ben-Ami et al. (2011) proposed that MSCs could alleviate the symptoms of systemic lupus erythematosus by inhibiting the activation and proliferation of T cells and reducing the production of pro-inflammatory cytokines. MSCs have shown similar therapeutic effects in autoimmune diseases such as rheumatoid arthritis and Crohn's disease. The positive results of these clinical trials provide a solid basis for the widespread use of MSCs in the treatment of autoimmune diseases.
Zaripova et al. (2023) point out that despite the great therapeutic potential of MSCs, there are still many challenges to their practical application. How to ensure the stable source and controllable quality of MSCs, how to optimize their culture and differentiation conditions, and how to ensure their long-term survival and stable function in vivo are all urgent problems to be solved. In addition, the specific mechanism of action of MSCs in the treatment of autoimmune diseases still needs more in-depth research and understanding.
4.2 Future directions of MSCs research
4.2.1 Improve survival rate and transplantation efficiency
Gomzikova et al. (2019) proposed that in order to improve the survival rate and transplantation efficiency of MSCs in vivo, researchers are exploring multiple strategies. One approach is to genetically engineer MSCs to express more molecules that contribute to cell survival and tissue integration, such as anti-apoptotic proteins and pro-angiogenesis factors. In addition, the use of biomaterials as carriers to protect MSCs from the attack of the immune system, while providing a suitable microenvironment to support their functional play in vivo, is also a current research focus.
4.2.2 Enhance the ability of immune regulation
Zaripova et al. (2023) believe that enhancing the immunomodulatory ability of MSCs is another important direction for future research. Preconditioning (preactivation) of MSCs to enhance their anti-inflammatory and immunomodulatory capacity prior to transplantation is one strategy. For example, the anti-inflammatory properties of MSCs can be enhanced by in vitro exposure to hypoxic or inflammatory cytokines. Researchers are also exploring ways to enhance the immunomodulatory effects of MSCs through molecular targeting and drug loading systems, such as the use of specific signaling molecular inhibitors or the release of immunomodulatory drugs, to achieve more precise and effective therapeutic effects.
Wang et al. (2014) believe that although MSCs face many challenges in the treatment of autoimmune diseases, their unique immunomodulatory properties and regenerative capacity provide a new therapeutic direction for the clinic. Through continuous research and technological innovation, it is expected that in the future, MSCs will be widely used in the treatment of autoimmune diseases in a safer and more effective way, bringing hope and improvement to patients.
4.2.3 Application of gene modification and cell engineering in improving the therapeutic effect of MSCs
Kong et al. (2009) believe that with the rapid development of gene editing technology, gene modification and cell engineering provide broad application prospects for improving the therapeutic effect of MSCs. In the future, gene editing technologies such as CRISPR-Cas9 can be used to precisely modify MSCs to enhance their immunomodulatory function, improve the therapeutic effect or extend their survival time. In addition, through the application of cell engineering techniques, such as induced pluripotent stem cell (iPS) technology, MSCs with specific functions can be produced on a large scale to meet clinical needs.
4.2.4 Combined application of MSCs with other therapeutic strategies
Peng et al. (2020) believe that MSCs have powerful immunomodulatory functions and can be combined with other therapeutic strategies, such as immunosuppressive drugs and cytokines, to achieve better therapeutic effects. In the future, we can study the combined application of MSCs and immunosuppressants to reduce the dosage and side effects of immunosuppressants; At the same time, the combined application of MSCs and cytokines can also be explored to enhance their immunomodulatory effects. In addition, MSCs can also be combined with other cell therapies (such as CAR T cells, NK cells, etc.) to form a comprehensive treatment regimen.
4.2.5 Development of novel biomaterials and tissue engineering methods
Ringden et al. (2022) believe that in order to optimize the transplantation and survival environment of MSCs, novel biomaterials and tissue engineering methods need to be developed. In the future, biological materials with biological activity, such as biomimetic materials and hydrogels, can be studied to simulate the in vivo environment and provide suitable growth conditions for MSCs. At the same time, we can also build tissues or organs with specific structures and functions through tissue engineering technology to provide a more ideal transplantation environment for MSCs.
4.2.6 Strengthen basic research and clinical trials
Huang et al. (2022) mentioned that in order to verify the efficacy and safety of MSCs in the treatment of autoimmune diseases, we need to strengthen basic research and clinical trials. In terms of basic research, we need to further study the biological characteristics of MSCs, immune regulatory mechanisms, and interaction mechanisms with other therapeutic strategies. In terms of clinical trials, we need to conduct large-scale, multi-center clinical trials to evaluate the efficacy and safety of MSCs treatment in different autoimmune diseases. At the same time, a sound follow-up system and database can be established to collect and analyze long-term efficacy and safety data. Through the development of these basic studies and clinical trials, it provides strong support for the wide application of MSCs in the treatment of autoimmune diseases.
Ben-Ami E., Berrih-Aknin S., and Miller A., 2011, Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases, Autoimmunity reviews, 10(7): 410-415.
https://doi.org/10.1016/j.autrev.2011.01.005
PMid:21256250
Castro-Manrreza M., and Montesinos J., 2015, Immunoregulation by Mesenchymal Stem Cells: Biological Aspects and Clinical Applications, Journal of Immunology Research, 2015: 394917.
https://doi.org/10.1155/2015/394917
PMid:25961059 PMCid:PMC4417567
Chen Y., Yu, Q., Hu, Y., and Shi Y., 2019, Current Research and Use of Mesenchymal Stem Cells in the Therapy of Autoimmune Diseases, Current stem cell research & therapy, 14(7): 579-582.
https://doi.org/10.2174/1574888X14666190429141421
PMid:31729289
Dalal J., Gandy K., and Domen J., 2012, Role of mesenchymal stem cell therapy in Crohn's disease, Pediatr Res., 71: 445-451.
https://doi.org/10.1038/pr.2011.56
PMid:22430380
Gao F., Chiu S., Motan D., Zhang Z., Chen L., Ji H., Tse H., F Q., and Lian Q., 2016, Mesenchymal stem cells and immunomodulation: current status and future prospects, Cell Death & Disease, 7(1): e2062.
https://doi.org/10.1038/cddis.2015.327
PMid:26794657 PMCid:PMC4816164
Gomzikova M., James V., and Rizvanov A., 2019, Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation, Frontiers in Immunology, 10: 2663.
https://doi.org/10.3389/fimmu.2019.02663
PMid:31849929 PMCid:PMC6889906
Huang Y., Wu, Q., and Tam, P., 2022, Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications, International Journal of Molecular Sciences, 23(17): 10023.
https://doi.org/10.3390/ijms231710023
PMid:36077421 PMCid:PMC9456387
Kong W.X., Jiang X.X., and Mao N., 2009, Immunomodulatory function of mesenchymal stem cells and the application of MSCs in the treatment of autoimmune diseases, Zhongguo Shiyan Xueyexue Zazhi (Chinese Journal of Experimental Hematology), 17(6): 1605-1608.
Munir H., and McGettrick H., 2015, Mesenchymal Stem Cell Therapy for Autoimmune Disease: Risks and Rewards, Stem Cells and Development, 24(18): 2091-2100.
https://doi.org/10.1089/scd.2015.0008
PMid:26068030
Peng X.F., and Chen F., 2020, Immunomodulatory functions of exosomes from mesenchymal stem cells and their use in autoimmune inflammatory diseases, Zhonghua Xibao yu Gaoxibao Zazhi (Chinese Journal of Cells and Stem cells), 10(4): 246-250.
Ringdén O., Moll G., Gustafsson B., Sadeghi B., Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-Versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome, Front Immunol., 13: 839844.
https://doi.org/10.3389/fimmu.2022.839844
PMid:35371003 PMCid:PMC8973075
Wang Y., Chen X.D., Cao W., and Shi Y.F., 2014, Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications, Nature Immunology, 15: 1009-1016.
https://doi.org/10.1038/ni.3002
PMid:25329189
Yi T., and Song S., 2012, Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Archives of Pharmacal Research, 35: 213-221.
https://doi.org/10.1007/s12272-012-0202-z
PMid:22370776
Zaripova L., Midgley A., Christmas S., Beresford M., Pain C., Baildam E., and Oldershaw R., 2023, Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases, International Journal of Molecular Sciences, 24(22): 16040.
https://doi.org/10.3390/ijms242216040
PMid:38003230 PMCid:PMC10671211
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Caixin Li
Related articles
. Mesenchymal stem cells (MSCs)
. Autoimmune diseases
. Immunomodulation
. Multi-directional differentiation
. Clinical trials
Tools
. Post a comment