Research Report
New hope in transplantation medicine: the role of mesenchymal stem cells in the prevention and treatment of GVHD 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 3
Received: 12 May, 2024 Accepted: 14 Jun., 2024 Published: 25 Jun., 2024
GVHD is a common complication after bone marrow transplantation, which can cause multiple system damage and seriously affect the quality of life and survival of patients. MSCs have become a hot topic in the treatment of GVHD in recent years due to their unique immune regulatory properties. This study explores the potential role of mesenchymal stem cells (MSCs) in the prevention and treatment of graft-versus-host disease (GVHD), and analyzes the efficacy and safety of MSCs in GVHD treatment. The results indicate that MSCs can effectively improve GVHD symptoms, prolong patient survival, and have fewer adverse reactions. MSCs, as an innovative therapy, have shown great potential in the prevention and treatment of GVHD. This study aims to clarify the efficacy, safety, and mechanism of action of MSCs in the prevention and treatment of GVHD by comprehensively analyzing existing research data, providing theoretical support and practical guidance for clinical application.
In the past few decades, transplant medicine has made significant progress, providing new treatment options for many previously difficult to treat diseases. With the development of technology and a deeper understanding of transplant biology, transplantation has become an effective way to reconstruct damaged tissue and organ function. However, despite significant technological advancements, post transplant complications, especially graft-versus-host disease (GVHD), remain one of the main limiting factors for transplant success rates (Kuba and Raida, 2018).
GVHD is a complex immune mediated response that typically occurs after allogeneic hematopoietic stem cells or organ transplantation. It is divided into acute and chronic forms, each with its specific clinical manifestations and onset time. Acute GVHD usually occurs within the first 100 days after transplantation, characterized mainly by skin, liver, and gastrointestinal damage; Chronic GVHD may occur 100 days later, with more diverse manifestations that can affect multiple organs and tissues (Lee, 2017). The occurrence of GVHD not only increases patient pain and treatment costs, but also seriously affects the quality of life and long-term survival rate after transplantation.
In the process of seeking effective prevention and treatment methods for GVHD, mesenchymal stem cells (MSCs) have received widespread attention due to their unique immune regulatory ability. MSCs are a type of pluripotent stem cells with broad differentiation potential, which can be isolated from bone marrow, adipose tissue, and various other tissues (Ouryazdanpanah et al., 2018). In addition to being able to differentiate into multiple cell types, MSCs also have the ability to regulate immune responses, promote tissue repair, and suppress inflammation, making them demonstrate enormous potential in regenerative medicine and immunomodulatory therapies. Especially in the prevention and treatment of GVHD, the application research of MSCs has brought new hope to the field of transplant medicine.
This study explores the application of mesenchymal stem cells in transplantation medicine, particularly their role and potential mechanisms in the prevention and treatment of GVHD. By gaining a deeper understanding of the characteristics and mechanisms of action of MSCs, it is expected to provide new strategies for the management of postoperative complications, thereby improving the prognosis and quality of life of transplant patients.
1 The Pathogenesis and Influencing Factors of GVHD
1.1 The pathogenesis of GVHD
Graft-versus-host disease (GVHD) is a complex immune mediated response that may occur after allogeneic hematopoietic stem cell or organ transplantation. Its pathogenesis mainly involves three stages: activation of the host immune system, activation and proliferation of immune cells, and ultimately attack on the host tissue. During the transplantation process, the donor's T cells are activated and rapidly proliferate upon contact with the host's allogeneic human leukocyte antigen (HLA). These T cells release a large amount of cytokines, such as interferon γ And tumor necrosis factor α. These factors not only enhance the activity of T cells, but also attract other immune cells such as macrophages to participate in the response. Activated immune cells then attack the host's skin, liver, and gastrointestinal tissues, leading to clinical manifestations of GVHD.
Ferrara and Chaudhry (2018) described how acute GVHD targets the crypts responsible for intestinal mucosal self-renewal in the intestine (Figure 1). The latest advances in the recognition and cultivation of intestinal stem cells have improved scientists' understanding of the interactions between the microbiota and the immune system (including innate and adaptive), which are key to the pathophysiology of GVHD. Serum biomarkers obtained from the gut are the most predictive of long-term outcomes of GVHD, and these biomarkers focus on cellular elements that serve as protective barriers and targets for GVHD.
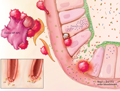 Figure 1 Early acute GI GVHD (Ferrara and Chaudhry, 2018) |
Toubai et al. (2008) discussed and compared the pathophysiology of acute and chronic GVHD. Acute GVHD typically manifests within 100 days after HSCT (hematopoietic stem cell transplantation), triggered by a response of donor T cells to host polymorphic histocompatibility antigens. Chronic GVHD usually manifests after 100 days and has certain characteristics of autoimmune diseases. It may be newly developed, or it may be a continuation of acute GVHD after resolution.
The pathogenesis of GVHD includes the T cell-mediated characteristics of the disease, interactions with the microbiota, and differences between acute and chronic forms. Through these studies, a more comprehensive perspective on the complexity and management strategies of the disease can be obtained.
1.2 Factors affecting the occurrence of GVHD
The occurrence and severity of GVHD are influenced by various factors. Among them, HLA typing differences between donors and recipients are an important factor; Transplantation with HLA matching typically has a lower risk of GVHD. In addition, the gender, age, and genetic background of the donor and recipient can also affect the risk of GVHD. For example, transplantation from young female donors to older male recipients may increase the incidence of GVHD. The pre-transplantation treatment plan, including the intensity of chemotherapy and/or radiation therapy, as well as the transplant treatment method, such as removing T cells, can also affect the occurrence of GVHD. In addition, post transplant infections and other complications may also increase the risk of GVHD.
Saliba et al. (2007) evaluated M D. The incidence, risk factors, clinical manifestations, and outcomes of hyperacute GVHD occurring within 14 days after transplantation were defined in the 809 consecutive HSCTs conducted by Anderson Cancer Center. Research has found that skin involvement is more common (88% compared to 44%) and more severe (Stage III-IV, 88% compared to 66%) in the hyperacute GVHD group compared to acute GVHD diagnosed after 14 days. Important risk factors include mismatched or unrelated donors, medulloblast transplantation regimens, more than 5 previous chemotherapy regimens, and donor recipient gender mismatch.
Jacobs et al. (2019) investigated the correlation between mood and quality of life in patients with chronic GVHD, and found that negative emotion oriented coping style, poor physical function and higher burden of symptoms were independently related to depressive symptoms. The study emphasizes the unmet physical and psychosocial needs of chronic GVHD patients and suggests the need to explore evidence-based interventions to improve quality of life and mood by targeting modifiable psychosocial structures identified in this study.
Wu et al. (2020) summarized the latest advances in the pathophysiology, prevention, and treatment of GVHD, as well as the application of traditional Chinese medicine in it. The study provides ideas and methods for exploring the mechanisms of traditional Chinese medicine and establishing new comprehensive treatment methods for GVHD. These studies indicate that the occurrence of GVHD is associated with multiple factors, including clinical and laboratory based factors, coping strategies, psychosocial factors, and treatment methods.
2 The Characteristics of Mesenchymal Stem Cells and Their Role in Immune Regulation
2.1 Source, types, and differentiation potential of MSCs
Mesenchymal stem cells (MSCs) are a type of pluripotent stem cells with significant medical application potential. They can be isolated and cultured from various tissues, including but not limited to bone marrow, adipose tissue, umbilical cord blood, placenta, and dental pulp. These MSCs from different sources exhibit differences in their cell phenotype, proliferation ability, and differentiation potential, but they generally express specific surface markers such as CD105, CD73, and CD90, while not expressing immune related surface molecules such as blood cell markers and HLA-DR. Bone marrow-derived MSCs (BM MSCs) were the earliest studied types and play a crucial role in maintaining hematopoietic stem cell function. In contrast, adipose tissue derived MSCs (AD MSCs) have attracted attention due to their ease of acquisition and high in vitro proliferation rate.
In addition, MSCs derived from perinatal tissues such as umbilical cord blood and placenta exhibit stronger proliferation ability and wider differentiation potential. Zhang and Chen (2012) pointed out that the human umbilical cord is a rich source of MSCs. Compared with MSCs from other sources, hUC MSCs have multiple advantages, including abundant sources, easy extraction, short doubling time, low immunogenicity, long-term survival after transplantation, and no ethical issues. Under suitable conditions, hUC MSCs can differentiate into neuroid cells and promote neural function recovery in various animal models of neurological diseases.
One of the most remarkable characteristics of MSCs is their multidirectional differentiation ability. They can differentiate into various cell types such as bone cells, adipocytes, and chondrocytes under appropriate induction conditions, providing new therapeutic strategies for the repair and regeneration of damaged tissues. Kuroda et al. (2012) further found that MSCs can even differentiate into nerve cells, liver cells, and myocardial cells under specific conditions, which broadens the application scope of MSCs in tissue engineering and regenerative medicine.
Through in-depth research and utilization of these characteristics of MSCs, scientists are committed to developing cell therapy methods based on MSCs to treat various diseases and improve the quality of life of patients.
2.2 The mechanism of action of MSCs in the immune system
Mesenchymal stem cells (MSCs) play a crucial immune regulatory role in the immune system, achieving their functions through a series of complex mechanisms. These mechanisms include direct and indirect regulation of various immune cells, thereby having a profound impact on immune responses. Firstly, MSCs are able to directly contact or secrete specific inhibitory factors, such as transforming growth factors β (TGF-β) and prostaglandin E2 (PGE2) is used to inhibit the proliferation and activation of T cells. This effect not only limits the spread of inflammatory reactions, but also promotes the generation of regulatory T cells (Tregs), thereby enhancing the immunosuppressive environment in the body.
In addition to regulating T cells, MSCs also affect B cell function by inhibiting B cell proliferation, maturation, and antibody secretion, thereby regulating humoral immune responses and reducing the production of autoantibodies. In addition, MSCs can also reduce the cytotoxic effects of natural killer cells (NK) and interferon γ (IFN-γ) by inhibiting the maturation and antigen-presenting ability of dendritic cells (DCs) and reducing T cell activation, the immune response can be regulated at both natural and adaptive immune levels.
Xiang et al. (2017) explored the application of MSCs in the treatment of glioma, emphasizing the potential of MSCs as cell therapeutic agents. The anti proliferative, anti-inflammatory, and anti apoptotic effects of MSCs have a protective effect on nerve cells, indicating their dual functions in regulating immune responses and supporting tissue regeneration.
Caplan and Correa (2011) proposed that MSCs can be released from their perivascular positions during injury, activated, and establish a regenerative microenvironment by secreting bioactive molecules and regulating local immune responses. These nutritional and immune regulatory activities indicate that MSCs can act as pharmacies for site regulation in the body.
The immune regulatory function of MSCs is not limited to inhibiting immune responses, but they can also promote tissue repair and regeneration by secreting various growth factors, such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), thus playing a dual role in immune-mediated diseases and tissue damage. By creating an immune tolerant microenvironment, MSCs have shown great potential in the treatment of autoimmune diseases, inflammatory diseases, and the prevention and treatment of graft-versus-host disease (GVHD) in transplant medicine.
3 The Application of 3 MSCs in Preventing GVHD
3.1 Research on MSCs for preventing GVHD in preclinical and clinical studies
In preclinical and clinical studies, mesenchymal stem cells (MSCs) have been widely studied as a potential strategy for preventing graft-versus-host disease (GVHD). These studies focus on evaluating the safety, efficacy, and how MSCs can prevent or reduce the severity of GVHD through immune regulatory mechanisms.
Preclinical studies, mainly conducted through animal models, have shown the positive effects of MSCs in preventing GVHD. In these studies, MSCs have been shown to reduce the release of inflammatory cytokines, inhibit the activation and proliferation of effector T cells, promote the formation of regulatory T cells, and thus alleviate the symptoms of GVHD. For example, studies in mouse models have shown that untreated mice exhibit more severe GVHD symptoms, including skin and liver damage, compared to mice treated with MSCs before and after transplantation.
Lim et al. (2017) found that MSCs significantly alleviate the clinical and pathological severity of acute skin sclerosing GVHD in a mouse model by inhibiting skin infiltration of immune effector cells. Research has found that MSCs can significantly reduce collagen production in the skin and consistently decrease Tgfb expression. In addition, MSCs inhibit the infiltration of immune effector cells by downregulating the expression of CCR4 and CCR8 on CD4+T cells and CCR1 expression on CD11b+monocytes/macrophages. These preclinical studies provide a foundation for the application of MSCs in human GVHD prevention strategies.
Based on the success of preclinical studies, clinical studies have begun to explore the application of MSCs in preventing human GVHD. These studies include small-scale early-stage trials and some larger randomized controlled trials. Clinical research results indicate that the infusion of MSCs is usually well tolerated by patients and can reduce the incidence and severity of GVHD. For example, Yao et al. (2023) explored the role of mesenchymal stem cells in preventing graft-versus-host disease and found that patients who used MSCs to prevent GVHD had fewer severe forms of acute GVHD. In addition, among patients in the MSCs treatment group, some long-term follow-up showed improved survival rates and quality of life.
However, despite many positive results, there is also some variability in clinical studies, partly influenced by factors such as different study designs, sources of MSCs, timing and dosage of administration. In addition, some studies have pointed out that although MSCs can alleviate GVHD symptoms in the short term, their long-term effects and potential side effects still need further research and monitoring. Pallua et al. (2010) found that GvHD significantly affects the role function, overall quality of life, fatigue, breathing difficulties, gastrointestinal side effects, concerns/anxiety, and skin problems of hematopoietic stem cell transplant survivors.
3.2 Mechanisms of MSCs in preventing GVHD
Mesenchymal stem cells (MSCs) play a crucial role in preventing graft-versus-host disease (GVHD), and their mechanism mainly depends on their immune regulatory ability. MSCs intervene in immune responses through multiple pathways, significantly reducing the occurrence and severity of GVHD. Fiori et al. (2021) believe that they can inhibit the activation and proliferation of immune cells, especially T and B cells, mainly by secreting a series of anti-inflammatory and immune regulatory factors, such as transforming growth factor β (TGF-β), Prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), and indoleamine 2,3-dioxygenase (IDO). These factors directly act on immune cells, inhibiting their attack on host tissues.
In addition, MSCs also play a role by promoting the formation and function of regulatory T cells (Tregs), which are naturally occurring immunosuppressive cells that can effectively inhibit the development of GVHD. By enhancing the activity of Tregs, MSCs help establish immune tolerance and prevent excessive immune responses. Cuerquis et al. (2014) found that in terms of the balance of inflammatory factors, MSCs reduced pro-inflammatory factors such as tumor necrosis factor α (TNF)- α) And interferon γ (IFN-γ) At the same time, it increases the secretion of anti-inflammatory cytokines such as interleukin-10 (IL-10), which reduces the inflammatory response.
Hemeda et al. (2010) found that MSCs also affect the migration of immune cells by regulating the expression of chemical factors and their receptors, reducing their accumulation in common target organs of GVHD such as the liver, intestines, and skin. In addition to immune regulatory effects, MSCs can also secrete growth factors to promote the repair and regeneration of damaged tissues, which is particularly important for reducing tissue damage caused by GVHD.
3.3 Current challenges and future research directions
Mesenchymal stem cells (MSCs) have shown enormous therapeutic potential in preventing graft-versus-host disease (GVHD), but their clinical application still faces multiple challenges. Firstly, there are prominent issues with the consistency and reproducibility of research results, which are mainly attributed to differences in the sources, preparation methods, timing of administration, and dosage of MSCs. In addition, although MSCs are generally considered safe, further research is needed on their long-term safety and potential side effects, such as potential tumor promoting effects or impact on host immune surveillance. Meanwhile, the specific mechanism of MSCs in immune regulation is not yet fully understood, and a deeper understanding of the mechanism is crucial for optimizing treatment strategies. Economic costs and ethical issues are also important factors that must be considered when implementing the clinical application of MSCs, especially when dealing with and using MSCs derived from embryos.
The future research direction should focus on optimizing the preparation and application procedures of MSCs, including ensuring the safety, efficacy, and consistency of treatment through standardized processes. This involves determining the optimal cell source, dosage, timing and route of administration. Further mechanism research, especially in-depth exploration of the interaction between MSCs and the host immune system, will provide important information for improving treatment efficacy. Meanwhile, implementing large-scale, multicenter, randomized controlled clinical trials will help validate the efficacy and safety of MSCs in preventing GVHD. Exploring the combined application of MSCs with other prevention or treatment methods for GVHD is also a promising research direction. In addition, finding ways to reduce costs and addressing ethical issues are also key to promoting the widespread application of MSCs in transplant medicine.
4 The Application of Mesenchymal Stem Cells in the Treatment of GVHD
4.1 Clinical trials and efficacy evaluation of mesenchymal stem cells in GVHD treatment
In the past few years, mesenchymal stem cells (MSCs) have shown significant therapeutic potential in the treatment of graft-versus-host disease (GVHD), attracting widespread clinical research interest. Multiple clinical trials mainly focus on evaluating the safety and effectiveness of MSCs in treating acute and chronic GVHD, while examining their potential improvements in patient survival and quality of life. The current research results indicate that the application of MSCs in the treatment of GVHD is generally considered safe and has not caused significant adverse events such as allergic reactions, acute toxicity, or increased risk of infection. However, for the potential risks of MSCs promoting tumor growth, long-term safety still needs to be continuously monitored and evaluated.
In terms of effectiveness, MSCs have shown varying degrees of effectiveness in alleviating acute and chronic GVHD symptoms. Especially for some patients with refractory acute GVHD, MSCs treatment can significantly reduce symptom severity and improve survival rate. Resnick et al. (2013) reported the results of MSC treatment in 50 patients with acute GVHD. These patients did not show any response after receiving hormone therapy for at least 3 days (≥1 mg/kg/day). This study found that among patients receiving MSC treatment, 33% achieved complete remission and 52% achieved partial remission, demonstrating the potential effectiveness of MSC treatment.
Although there is relatively limited data on chronic GVHD, preliminary studies have also shown that MSCs have potential therapeutic benefits for some patients. In addition, MSCs treatment has shown positive effects in improving the long-term survival rate and quality of life of GVHD patients. As shown in the study by Wen et al. (2010), infusion of ex vivo expanded MSCs may be a safe and effective rescue therapy for patients with refractory chronic GVHD. 19 stubborn chronic GVHD patients received MSCs treatment from volunteer bone marrow, of which 14 patients (73.7%) had a good response to MSCs treatment, achieving complete remission (CR) or partial remission (PR). After treatment, some surviving patients were able to reduce immunosuppressive agents to below 50% of the initial dose, and 5 patients were able to completely discontinue immunosuppressive agents.
However, there are significant individual differences in the effectiveness of MSCs treatment, which may be related to various factors such as the treatment plan (such as the source, dosage, and timing of MSCs) and the patient's own conditions. Therefore, future research will need to focus on optimizing treatment plans, determining the most suitable cell dose, infusion timing, and treatment frequency. Exploring the mechanism of action of MSCs and developing biomarkers for predicting treatment outcomes is of great significance for improving treatment outcomes and developing personalized treatment strategies.
4.2 Mechanism of action of MSCs in the treatment of GVHD
The mechanism of action of mesenchymal stem cells (MSCs) in the treatment of graft-versus-host disease (GVHD) involves their strong immune regulatory function and ability to promote tissue repair, which constitutes the multifaceted impact of MSCs in the treatment of GVHD. MSCs reduce attacks on host tissues by inhibiting the activation and proliferation of effector T cells, which is achieved through direct contact and secretion of anti-inflammatory cytokines such as TGF- β Implemented with PGE2. Meanwhile, MSCs also promote the generation and function of regulatory T cells (Tregs), which play a crucial role in maintaining immune tolerance and inhibiting GVHD. In addition, the impact of MSCs on B cell function is achieved by inhibiting B cell proliferation and antibody production, alleviating autoimmune reactions, while also regulating the function of natural killer (NK) cells and dendritic cells (DC), further reducing the intensity of immune responses.
In addition to its immunomodulatory effects, MSCs also promote the repair and regeneration of damaged tissues by secreting various growth factors, particularly in alleviating long-term tissue damage caused by GVHD. Qi et al. (2017) discussed how MSCs interact with the immune system to promote tissue repair, emphasizing the bidirectional regulation between MSCs and immune responses in stem cell-based tissue repair processes.
MSCs can also regulate inflammatory responses by secreting anti-inflammatory cytokines and inhibiting pro-inflammatory cytokines such as IL-10 and TNF- α Inhibitors are used to alleviate the inflammatory damage caused by GVHD. Jiang and Xu (2019) discussed that MSCs promote inflammation when the immune system is inactive, and suppress inflammation when the immune system is overactive to avoid self injury. They explored the transition of MSCs from pro-inflammatory to anti-inflammatory phenotypes, effectively regulating their ability to regulate immune responses.
Le and Ringdén (2005) reviewed the immunobiology of MSCs, their role in enhancing hematopoietic transplantation implantation, and their use as a preventive and therapeutic approach for acute and chronic GVHD. In the treatment of chronic GVHD, MSCs demonstrate their unique potential in combating chronic GVHD by reducing fibroblast activation and inhibiting collagen deposition, thereby alleviating the fibrosis process.
4.3 Advantages and limitations of mesenchymal stem cell therapy for GVHD
The application of mesenchymal stem cells (MSCs) in the treatment of graft-versus-host disease (GVHD) has demonstrated significant advantages, but also faces a series of limitations. MSCs, through their excellent immune regulatory ability, can effectively suppress excessive immune responses and promote immune tolerance, which is particularly important in the treatment of immune mediated diseases such as GVHD. In addition, MSCs can promote the repair and regeneration of damaged tissues, which is crucial for the recovery of GVHD patients. The wide range of sources from various tissues such as bone marrow, fat, umbilical cord blood, and placenta, as well as their low immunogenicity, reduces the risk of immune rejection, making MSCs suitable for allogeneic transplantation. In addition, a large number of clinical trials have proven the relative safety of MSCs application, and no serious adverse events have been observed.
Blanc et al. (2008) found that mesenchymal stem cell therapy is effective in severe cases, and MSCs have been successful in treating severe acute GVHD resistant to steroids, with a considerable number of patients achieving complete or partial remission. Kebriaei et al. (2009) found that MSCs are safe and well tolerated in treatment, with few side effects occurring during and immediately after infusion.
However, the application of MSCs in the treatment of GVHD also has limitations. The individual differences in treatment effectiveness are significant and are influenced by factors such as the patient's specific situation, the source and quality of MSCs, and the treatment plan. Although MSCs treatment is relatively safe in the short term, its long-term safety, especially regarding the potential risks of promoting tumor growth (Figure 2), still requires more research and long-term monitoring. In addition, the immune regulatory mechanisms of MSCs are complex and not fully understood, which limits the optimization of treatment strategies. The cost issue is also a challenge that cannot be ignored, as high acquisition, cultivation, and application costs may limit its application in resource limited areas. Finally, the use of embryonic derived MSCs may raise ethical and legal issues.
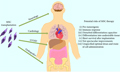 Figure 2 The scheme of potential risks of adverse events during MSC transplantation (Lukomska et al., 2019) |
Lukomska et al. (2019) believe that the efficacy of MSCs varies greatly among patients, with some patients having no response and long-term benefits not yet fully determined. Kim et al. (2013) argue that the management of MSCs is complex, and the timing and conditions of MSCs management are crucial, especially in the typical pro-inflammatory environment of acute GVHD. This makes the timing of administration crucial for the therapeutic effect. In summary, although MSCs provide a powerful and promising approach for the treatment of severe GVHD, broader clinical trials and long-term follow-up studies are needed to fully understand their efficacy and safety profile.
5 Future Research Directions and Prospects
With a deeper understanding of the mechanism of action of mesenchymal stem cells (MSCs) in the treatment of graft-versus-host disease (GVHD), optimizing treatment strategies has become a focus of future research. On the one hand, research will focus on the extraction, amplification, and application of standardized MSCs to ensure consistency and reproducibility of treatment. On the other hand, customized treatment plans that adjust the dosage, infusion frequency, and timing of MSCs based on the specific condition of the patient (such as disease stage, immune system status, etc.) may become the key to improving treatment effectiveness. Meanwhile, exploring the combined application of MSCs with other treatment methods (such as immunosuppressants, biologics, etc.) to achieve synergistic effects is also an important direction for future research.
In addition to its application in GVHD treatment, other potential applications of MSCs in transplant medicine are also worth paying attention to. For example, MSCs can be used to improve tissue fusion and functional recovery after organ transplantation due to their ability to promote tissue repair and regeneration (Barreca et al., 2020). In addition, the immunomodulatory properties of MSCs make them promising therapeutic tools for treating autoimmune diseases and promoting transplant tolerance. Future research will also explore the application of MSCs in preventing post transplant infections, promoting angiogenesis, and improving long-term graft survival.
In the future, transplant medicine will place greater emphasis on individualized and precision medicine to improve transplant success rates and patient quality of life. MSCs, as a multifunctional cell therapy tool, play an increasingly important role in this process. With further research on the biological characteristics of MSCs and the accumulation of clinical application experience, it is expected to develop more effective and safe basic treatment plans for MSCs. In addition, the combination with regenerative medicine, tissue engineering, and immunomodulatory therapy will further expand the application scope of MSCs in transplant medicine (Canceda et al., 2023). Ultimately, by comprehensively utilizing cell therapy techniques such as MSCs, future transplant medicine is expected to achieve better harmonious coexistence between the transplant and the host, bringing more effective treatment options and higher quality of life to patients.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Barreca M., Cancemi P., and Geraci F., 2020, Mesenchymal and Induced pluripotent stem cells-derived extracellular vesicles: the new frontier for regenerative medicine?. Cells, 9(5): 1163.
https://doi.org/10.3390/cells9051163
PMid:32397132 PMCid:PMC7290733
Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M., Remberger M., Dini G., Egeler R., Bacigalupo A., Fibbe W., and Ringdén O., 2008, Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study, The Lancet, 371: 1579-1586.
https://doi.org/10.1016/S0140-6736(08)60690-X
Le B., and Ringdén O., 2005, Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation.. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation, 11(5): 321-334.
https://doi.org/10.1016/j.bbmt.2005.01.005
PMid:15846285
Cancedda R., Murphy J., and Griensven M., 2023, Editorial: Insights in tissue engineering and regenerative medicine 2021: Novel developments, current challenges, and future perspectives, Frontiers in Bioengineering and Biotechnology, 10.
https://doi.org/10.3389/fbioe.2022.1125027
PMid:36714627 PMCid:PMC9877226
Caplan A., and Correa D., 2011, The MSC: an injury drugstore, Cell stem cell, 9(1): 11-15.
https://doi.org/10.1016/j.stem.2011.06.008
PMid:21726829 PMCid:PMC3144500
Cuerquis J., Romieu-Mourez R., François M., Routy J., Young Y., Zhao J., and Eliopoulos N., 2014, Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation.. Cytotherapy, 16(2): 191-202.
https://doi.org/10.1016/j.jcyt.2013.11.008
PMid:24438900
Ferrara J., and Chaudhry M., 2018, GVHD: biology matters, Blood advances, 2(22): 3411-3417.
https://doi.org/10.1182/bloodadvances.2018020214
PMid:30482771 PMCid:PMC6258915
Fiori A., Uhlig S., Klüter H., and Bieback K., 2021, Human adipose tissue-derived mesenchymal stromal cells inhibit CD4+ T cell proliferation and induce regulatory T Cells As Well as CD127 expression on CD4+CD25+ T cells. Cells, 10(1): 58.
https://doi.org/10.3390/cells10010058
PMid:33401501 PMCid:PMC7824667
Hemeda H., Jakob M., Ludwig A., Giebel B., Lang S., and Brandau S., 2010, Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells, Stem cells and development, 19(5): 693-706.
https://doi.org/10.1089/scd.2009.0365
PMid:20067407
Jacobs J., Fishman S., Sommer R., Sereno I., Fenech A., Jankowski A., Traeger L., Greer J., Vanderklish J., Hunnewell C., Saylor M., Chen Y., Spitzer T., DeFilipp Z., Temel J., and El-Jawahri A., 2019, Coping and modifiable psychosocial factors are associated with mood and quality of life in patients with chronic graft-versus-host-disease (GVHD), Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation, 25(11): P2234-2242.
https://doi.org/10.1016/j.bbmt.2019.06.024
PMid:31260800
Jiang W., and Xu J., 2019, Immune modulation by mesenchymal stem cells, Cell Proliferation, 53(1): e12712.
https://doi.org/10.1111/cpr.12712
PMid:31730279 PMCid:PMC6985662
Kebriaei P., Isola L., Bahceci E., Holland K., Rowley S., Mcguirk J., Devetten M., Jansen J., Herzig R., Schuster M., Monroy R., and Uberti J., 2009, Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease, Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation, 15(7): 804-811.
https://doi.org/10.1016/j.bbmt.2008.03.012
PMid:19539211
Kim N., Im K., Lim J., Jeon E., Nam Y., Kim E., and Cho S., 2013, Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice, Annals of Hematology, 92: 1295-1308.
https://doi.org/10.1007/s00277-013-1796-z
PMid:23722500
Kuba A., and Raida L., 2018, Graft versus host disease: from basic pathogenic principles to DNA damage response and cellular senescence, Mediators of Inflammation, 2018: 9451950.
https://doi.org/10.1155/2018/9451950
PMid:29785172 PMCid:PMC5896258
Kuroda Y., Kitada M., Wakao S., and Dezawa M., 2011, Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Archivum Immunologiae et Therapiae Experimentalis, 59: 369-378.
https://doi.org/10.1007/s00005-011-0139-9
PMid:21789625
Lee S., 2017, Classification systems for chronic graft-versus-host disease, Blood, 129(1): 30-37.
https://doi.org/10.1182/blood-2016-07-686642
PMid:27821503 PMCid:PMC5216262
Lim J., Ryu D., Lee S., Park G., and Min C., 2017, Mesenchymal stem cells (MSCs) attenuate cutaneous sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model, The Journal of Investigative Dermatology, 137(9): 1895-1904.
https://doi.org/10.1016/j.jid.2017.02.986
PMid:28526296
Lukomska B., Stanaszek L., Zuba-Surma E., Legosz P., Sarzyńska S., and Drela K., 2019, Challenges and controversies in human mesenchymal stem cell therapy, Stem Cells International, 2019: 9628536.
https://doi.org/10.1155/2019/9628536
PMid:31093291 PMCid:PMC6481040
Ouryazdanpanah N., Dabiri S., Derakhshani A., Vahidi R., and Farsinejad A., 2018, Peripheral blood-derived mesenchymal stem cells: growth factor-free isolation, molecular characterization and differentiation, Iranian Journal of Pathology, 13: 461-466.
Pallua S., Giesinger J., Oberguggenberger A., Kemmler G., Nachbaur D., Clausen J., Kopp M., Sperner-Unterweger B., and Holzner B., 2010, Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation, Bone Marrow Transplantation, 45: 1534-1539.
https://doi.org/10.1038/bmt.2010.5
PMid:20228854
Qi K., Li N., Zhang Z., and Melino G., 2017, Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response, Cellular immunology, 326: 86-93.
https://doi.org/10.1016/j.cellimm.2017.11.010
PMid:29221689
Resnick I., Barkats C., Shapira M., Stepensky P., Bloom A., Shimoni A., Mankuta D., Varda‐Bloom N., Rheingold L., Yeshurun M., Bielorai B., Toren A., Zuckerman T., Nagler A., and Or R., 2013, Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC), American journal of blood research, 3(3): 225-238.
Saliba R., Lima M., Giralt S., Andersson B., Khouri I., Hosing C., Ghosh S., Neumann J., Hsu Y., Jésus J., Qazilbash M., Champlin R., and Couriel D., 2007, Hyperacute GVHD: risk factors, outcomes, and clinical implications, Blood, 109(7): 2751-2758.
https://doi.org/10.1182/blood-2006-07-034348
PMid:17138825
Toubai T., Sun Y., and Reddy P., 2008, GVHD pathophysiology: is acute different from chronic? Best practice & research. Clinical haematology, 21(2): 101-117.
https://doi.org/10.1016/j.beha.2008.02.005
PMid:18503979
Weng J., Weng J., Du X., Du X., Geng S., Geng S., Peng Y., Wang Z., Lu Z., Lu Z., Wu S., Wu S., Luo C., Luo C., Guo R., Guo R., Ling W., Ling W., Deng C., Deng C., Liao P., Liao P., and Xiang A., 2010, Mesenchymal stem cell as salvage treatment for refractory chronic GVHD, Bone Marrow Transplantation, 45: 1732-1740.
https://doi.org/10.1038/bmt.2010.195
PMid:20818445 PMCid:PMC3035976
Wu X., Zhuang H., Zhao Y., Yu X., Dai T., and Gao R., 2020, Chinese medicine treatment on graft-versus-host disease after allogeneic hematopoietic stem cell transplantation, Chinese Journal of Integrative Medicine, 26: 324-329.
https://doi.org/10.1007/s11655-020-3252-y
PMid:32350801
Xiang B., Chen L., Wang X., and Xiang C. 2017, Mesenchymal stem cells as therapeutic agents and in gene delivery for the treatment of glioma, Journal of Zhejiang University-SCIENCE B, 18: 737-746.
https://doi.org/10.1631/jzus.B1600337
PMCid:PMC5611545
Yao H., Wang X., Huang R., Fu H., Lin R., Feng Y., Deng X., Chen T., Zhu L., Liu J., Liu Y., Zhao L., Wang L., Zhang C., Kong P., Gao L., Gao L., Liu Q., Zhang X., and Zhang X., 2023, A prospective, multi-center, randomized, controlled clinical trial to explore the role of mesenchymal stem cells in preventing graft versus host disease, Blood, 42(1): 3556.
https://doi.org/10.1182/blood-2023-189104
Zhang D.S., and Chen Q., 2012., The Latest Application Progress of Human Umbilical Cord-Derived Mesenchymal Stem Cells (hUC-MSCs) in Neurological Diseases, nternational Journal of Psychiatry and Neurology, 1(3): 17-21.
https://doi.org/10.12677/IJPN.2012.13005
.png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Caixin Li
Related articles
. Mesenchymal stem cell
. Transplantation medicine
. Graft-versus-host disease
. Immune regulation
. Treatment strategies
Tools
. Post a comment