Research Perspective
Unraveling the Gut-Brain Axis: The Potential of Engineered Synthetic Microbial Communities in Modulating Neurotransmitter Production and Mental Health 
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 2
Received: 03 Mar., 2024 Accepted: 14 Apr., 2024 Published: 22 Apr., 2024
This study delves into the intricate communication network between the gastrointestinal tract and the central nervous system, known as the gut-brain axis, and its significant impact on mental health. The study focuses on the potential of engineered synthetic microbial consortia (SynComs) in regulating neurotransmitter production and promoting mental well-being. By examining recent advancements in synthetic biology and multi-omics technologies, it highlights the substantial improvements in SynComs' ability to precisely target neurochemical pathways. The integration potential of personalized SynComs in the treatment of mental health disorders is emphasized, offering a promising alternative to traditional therapies. Additionally, the study discusses the challenges related to technology, safety, ethics, and regulation, providing a comprehensive overview of the current state and future prospects of SynComs in clinical applications. The importance of interdisciplinary collaboration in advancing SynCom research is underscored, calling for continued efforts to fully realize its therapeutic potential. This study demonstrates the transformative potential of SynComs in the field of mental health, providing a theoretical foundation for innovative and personalized therapeutic strategies.
The gut-brain axis, a complex bidirectional communication network between the gastrointestinal tract and the central nervous system, has garnered significant interest for its role in regulating mental health. Emerging research has highlighted how gut microbiota can influence brain function through various pathways, including immune modulation, hormone secretion, and direct microbial metabolite production. Understanding this intricate connection is crucial for developing novel therapeutic strategies for mental health disorders (Huang and Wu, 2021).
The gut-brain axis (GBA) involves multiple pathways, including neural, hormonal, and immune mechanisms, which facilitate the interaction between gut microbiota and brain function (Petra et al., 2015; Cryan et al., 2019; Socała et al., 2021). The gut microbiota, comprising trillions of microorganisms, plays a crucial role in maintaining homeostasis and influencing various physiological processes, including neural development, neurotransmission, and behavior (Martin et al., 2018; Cryan et al., 2019; Socała et al., 2021). Emerging evidence suggests that alterations in the gut microbiota composition can significantly impact mental health, contributing to the pathogenesis and progression of neuropsychiatric and neurological disorders such as depression, anxiety, schizophrenia, autism spectrum disorders, and Parkinson's disease (Huang and Wu, 2021; Margolis et al., 2021; Socała et al., 2021).
Synthetic microbial communities (SynComs) are engineered consortia of microorganisms designed to perform specific functions within a host environment. These communities offer a promising approach to modulate the gut microbiota and, consequently, the gut-brain axis. By precisely controlling the composition and metabolic activities of SynComs, researchers aim to influence neurotransmitter production and other biochemical pathways that affect brain function and mental health (Liu et al., 2015). SynComs can be tailored to produce specific metabolites, such as short-chain fatty acids, neurotransmitters, and other signaling molecules, which can interact with the central nervous system through various routes, including the vagus nerve, immune system, and endocrine pathways (Petra et al., 2015; Wiley et al., 2017; Martin et al., 2018). This targeted modulation holds potential for developing novel therapeutic strategies for mental health disorders.
This study article aims to deeply explore the potential of engineered synthetic microbial communities (SynComs) in regulating neurotransmitter production and improving mental health. By comprehensively analyzing existing research on the gut-brain axis and the impact of the gut microbiota on neuropsychiatric disorders, this article reveals how SynCom interventions can influence brain function and behavior. Key findings from preclinical and clinical studies are highlighted, mechanisms of gut-brain communication are discussed, and gaps in the current knowledge base are identified to guide future research. This study emphasizes the importance of SynComs as an innovative and promising therapeutic approach for the prevention and treatment of mental health issues, providing a theoretical foundation for future research and clinical practice.
1 The Gut-Brain Axis: A Complex Communication Network
1.1 Definition and components of the Gut-Brain axis
The gut-brain axis (GBA) is a bidirectional communication network that links the gastrointestinal (GI) tract and the central nervous system (CNS). This axis encompasses various components, including the enteric nervous system (ENS), the autonomic nervous system (ANS), the hypothalamic-pituitary-adrenal (HPA) axis, and the gut microbiota (Martin et al., 2018; Cryan et al., 2019; Hattori and Yamashiro, 2021). The ENS, often referred to as the "second brain", resides within the intestinal wall and communicates with the brain via the vagus nerve and other neural pathways (Hattori and Yamashiro, 2021). The ANS, comprising sympathetic and parasympathetic branches, modulates gut motility, secretion, and permeability, thereby influencing the gut microbiota (Martin et al., 2018). The HPA axis plays a crucial role in stress responses, linking the gut and brain through hormonal signaling (Hattori and Yamashiro, 2021). Collectively, these components form a complex network that maintains homeostasis and influences both gut and brain functions.
1.2 Mechanisms of communication between the gut and the brain
The communication between the gut and the brain occurs through multiple mechanisms, including neural, endocrine, and immune pathways (Figure 1). The vagus nerve is a primary neural conduit for gut-brain communication. It transmits signals from the gut to the brain and vice versa, playing a crucial role in regulating gut motility, secretion, and immune responses. The ENS also communicates directly with the CNS through intrinsic primary afferent neurons that detect changes in the gut environment (Bistoletti et al., 2020).
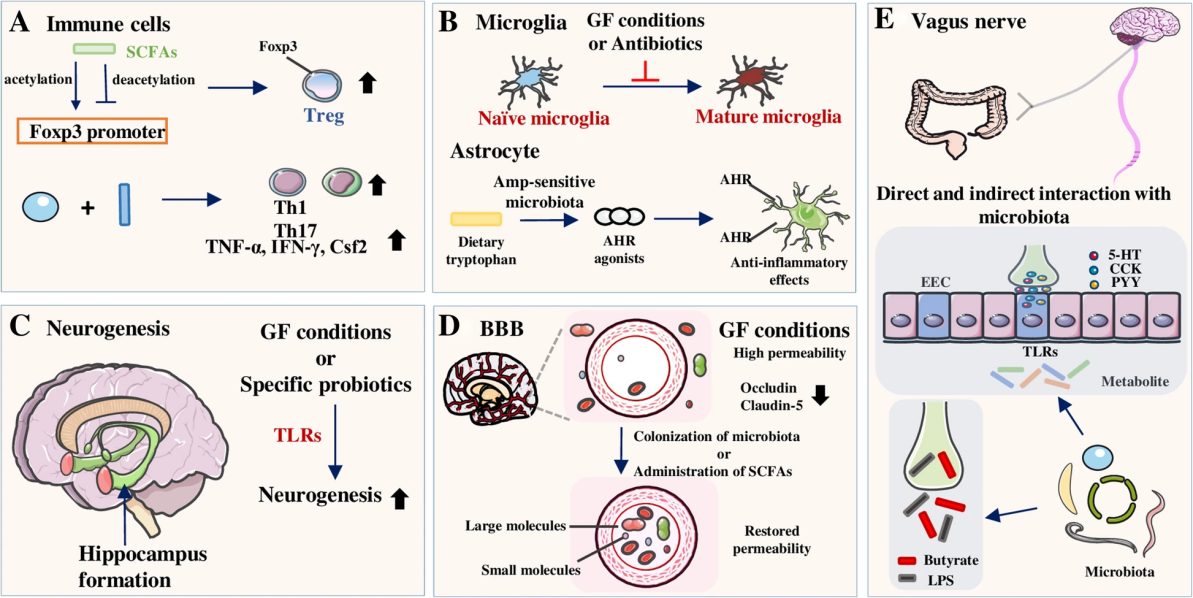 Figure 1 The complex interactions between the gut microbiota and the gut-brain axis (Adapted from Ma et al., 2019) Image caption: A shows that short-chain fatty acids (SCFAs) promote the generation of regulatory T cells (Tregs) by modulating the Foxp3 promoter, while inhibiting the formation of pro-inflammatory T cells; B explains that under germ-free conditions or with antibiotic use, the maturation of microglia is inhibited, affecting the anti-inflammatory effects in the nervous system; C indicates that specific probiotics can promote neurogenesis in the hippocampus; D demonstrates that under germ-free conditions, the permeability of the blood-brain barrier (BBB) increases, and the colonization of microbiota or the use of SCFAs can restore its normal permeability; E emphasizes that the vagus nerve interacts with the gut microbiota directly and indirectly, influencing brain function (Adapted from Ma et al., 2019) |
The research of Ma et al. (2019) shows the interaction between the gut microbiota and the gut-brain axis through various mechanisms, affecting the health of the central nervous system. Short-chain fatty acids regulate immune cells, promoting anti-inflammatory responses; microglial cell maturation is impaired under germ-free conditions, affecting neuroprotection; specific probiotics promote hippocampal neurogenesis; blood-brain barrier permeability increases under germ-free conditions but can be restored to normal function by microbial colonization or short-chain fatty acids; the vagus nerve directly and indirectly influences brain function by transmitting neural signals and metabolites. These mechanisms highlight the crucial role of gut microbiota in maintaining and regulating central nervous system health.
The HPA axis mediates the endocrine communication between the gut and the brain. In response to stress, the hypothalamus releases corticotropin-releasing hormone (CRH), which stimulates the pituitary gland to secrete adrenocorticotropic hormone (ACTH). ACTH then prompts the adrenal glands to release cortisol, a hormone that influences gut permeability and immune function. Additionally, gut hormones such as ghrelin, peptide YY, and glucagon-like peptide-1 (GLP-1) can signal the brain to regulate appetite and metabolism (Makris et al., 2021).
The immune system is a key player in the gut-brain axis. Gut-associated lymphoid tissue (GALT) monitors and responds to pathogens and other antigens in the gut. Immune cells release cytokines that can influence brain function and behavior. Chronic inflammation in the gut can lead to increased permeability of the blood-brain barrier, allowing immune signals to affect the brain directly. This interaction is bidirectional, as brain inflammation can also impact gut health and microbiota composition (Fung, 2020).
Additionally, microbial metabolites such as short-chain fatty acids (SCFAs), tryptophan metabolites, and peptidoglycans can modulate these communication pathways. These mechanisms collectively ensure a dynamic and responsive interaction between the gut and the brain.
1.3 Role of the gut microbiota in modulating these communication pathways
The gut microbiota, comprising trillions of microorganisms, plays a pivotal role in modulating the communication pathways of the GBA. The microbiota influences neural communication by interacting with the ENS and modulating vagal signaling (Cryan et al., 2019; Kuwahara et al., 2020). It also affects endocrine pathways by influencing the release of hormones such as serotonin, which is predominantly produced in the gut (Gao et al., 2019; Margolis et al., 2021). The immune system is another critical interface, with the gut microbiota modulating immune responses and maintaining the integrity of the intestinal barrier (Wang and Wang, 2016; Hattori and Yamashiro, 2021). Dysbiosis, or an imbalance in the gut microbiota, has been linked to various neurological and psychiatric disorders, including anxiety, depression, and autism (Wiley et al., 2017; Cryan et al., 2019). Probiotics, prebiotics, and other microbial-based interventions are being explored as potential therapeutic strategies to restore balance in the GBA and improve mental health outcomes (Wiley et al., 2017; Suganya and Koo, 2020). The intricate interplay between the gut microbiota and the GBA underscores the importance of maintaining a healthy and diverse microbial community for optimal brain function and mental health.
2 Neurotransmitter Production in the Gut
2.1 Overview of neurotransmitters produced in the gut
The gut is a significant site for the production of various neurotransmitters, including serotonin, dopamine, and gamma-aminobutyric acid (GABA). These neurotransmitters play critical roles in maintaining gastrointestinal (GI) homeostasis and facilitating communication along the gut-brain axis. Serotonin, for instance, is predominantly produced in the gut, with approximately 90% of the body's total serotonin synthesized by enterochromaffin cells in the gastrointestinal tract (Strandwitz, 2018; Liu and Huang, 2019). Dopamine, another critical neurotransmitter, is also produced in the gut, albeit in smaller quantities compared to the brain (Strandwitz, 2018; Bhatia et al., 2023). GABA, which plays a crucial role in inhibiting neural activity, is synthesized by certain gut bacteria, contributing to the overall pool of this neurotransmitter in the body (Strandwitz, 2018; Bhatia et al., 2023).
2.2 Microbial involvement in neurotransmitter synthesis and regulation
The gut microbiota plays a pivotal role in the synthesis and regulation of neurotransmitters. Various gut bacteria are capable of producing neurotransmitters directly. For example, certain strains of Lactobacillus and Bifidobacterium can produce GABA, while other bacteria can synthesize serotonin and dopamine (Strandwitz, 2018; Liu and Huang, 2019; Bhatia et al., 2023). Additionally, the gut microbiota can influence the availability of precursors for neurotransmitter synthesis. The metabolism of aromatic amino acids (AAAs) by gut bacteria can affect the levels of tryptophan and tyrosine, which are precursors for serotonin and dopamine, respectively (Gao et al., 2018; Gao et al., 2019). This microbial modulation of neurotransmitter precursors can subsequently impact neurotransmitter levels in the brain (Gao et al., 2018; Gao et al., 2019).
2.3 Impact of Gut-Derived neurotransmitters on brain function and mental health
Gut-derived neurotransmitters have a profound impact on brain function and mental health. The gut-brain axis facilitates bidirectional communication between the gut and the brain, allowing gut-derived neurotransmitters to influence central nervous system activities. For instance, alterations in gut microbiota composition have been linked to changes in brain neurotransmitter levels, which can affect mood and behavior (Chen et al., 2021; Huang and Wu, 2021; Socała et al., 2021). Studies have shown that Alterations in gut serotonin levels have been linked to conditions like irritable bowel syndrome (IBS) and depression. Serotonergic pathways in the gut can influence central serotonergic activity, impacting mood and behavior (Kumar et al., 2020). Increased GABA production in the gut, particularly under conditions like hepatic encephalopathy, can affect brain inhibition and lead to altered neural activity (Altaib et al., 2021). Moreover, interventions targeting the gut microbiota, such as the use of probiotics, prebiotics, or antibiotics, have been shown to alter neurotransmitter levels and improve symptoms of mental health disorders (Dinan and Cryan, 2017; Strandwitz, 2018; Huang and Wu, 2021).
3 Engineering Synthetic Microbial Communities (SynComs) for Neurotransmitter Modulation
3.1 Definition and principles of SynComs
Synthetic microbial communities (SynComs) are engineered consortia of microorganisms designed to perform specific functions that natural microbial communities may not efficiently achieve. These communities are constructed using principles of synthetic biology, which involves the design and assembly of genetic components to create new biological systems or reprogram existing ones. SynComs can be tailored to produce desired metabolites, including neurotransmitters, by incorporating specific microbial strains with known metabolic capabilities (Petra et al., 2015; Dinan and Cryan, 2017; Huang and Wu, 2021).
3.2 Techniques for engineering SynComs to enhance neurotransmitter production
Several techniques are employed to engineer SynComs for enhanced neurotransmitter production such as serotonin, dopamine, and gamma-aminobutyric acid (GABA):
1) Genetic Engineering: This involves the insertion, deletion, or modification of genes within microbial genomes to enhance their ability to produce specific neurotransmitters. For example, genes responsible for the production of serotonin, dopamine, and gamma-aminobutyric acid (GABA) can be inserted into microbial genomes to boost their production (Strandwitz, 2018; Baj et al., 2019; Huang and Wu, 2021).
2) Synthetic Biology: This broad field includes techniques such as the design of synthetic gene circuits and metabolic pathways that can control and optimize microbial behavior. Synthetic biology approaches enable the creation of complex genetic networks that regulate neurotransmitter production in response to environmental signals or internal cellular states (Kang et al., 2020).
3) Metabolic Engineering: This involves the optimization of metabolic pathways within microorganisms to increase the yield of neurotransmitters. By redirecting metabolic fluxes and eliminating competing pathways, the production of target neurotransmitters can be maximized (Dinan and Cryan, 2017; Margolis et al., 2021; Socała et al., 2021).
4) Quorum Sensing: By engineering quorum sensing systems, SynComs can coordinate their behavior and maintain stable population dynamics. This technique allows microbial communities to modulate neurotransmitter production collectively, based on cell density and environmental conditions (Scott and Hasty, 2016).
5) Mathematical Modeling and Computational Tools: Predictive models and computational tools are used to design and optimize SynComs. These models help in understanding the complex interactions within microbial consortia and guide the engineering of stable and functional communities (Zomorrodi and Segrè, 2016).
3.3 Advantages of using engineered SynComs over natural microbial communities
Engineered SynComs offer several advantages over natural microbial communities:
1) Predictability and Control: SynComs are designed to have predictable behaviors and functions, reducing the variability often seen in natural communities. This predictability is crucial for therapeutic applications where consistent outcomes are necessary (Karkaria et al., 2021).
2) Targeted Functionality: SynComs can be engineered to perform specific functions, such as the production of particular neurotransmitters, which might not be achievable with natural microbial communities. This targeted functionality allows for precise interventions in the gut-brain axis (Kang et al., 2020).
3) Stability: Engineered communities can be designed to be more stable and resilient to environmental changes compared to natural communities. This stability ensures that the desired functions are maintained over time and under varying conditions (Scott and Hasty, 2016).
4) Scalability and Reproducibility: SynComs can be scaled up and reproduced consistently, which is advantageous for clinical and industrial applications. This scalability ensures that large populations can be managed and utilized effectively for therapeutic purposes (Zomorrodi and Segrè, 2016).
5) Safety: Engineered SynComs can be designed to minimize the risk of pathogenicity and unwanted side effects. By using non-pathogenic strains and incorporating safety switches, the potential for adverse effects can be reduced (Dinan and Cryan, 2017; Strandwitz, 2018; Huang and Wu, 2021).
4 Applications of SynComs in Mental Health
4.1 SynComs for anxiety and depression management
Synthetic microbial communities (SynComs) have shown potential in managing anxiety and depression by modulating the gut-brain axis. The gut microbiome plays a crucial role in the production of neurotransmitters such as serotonin and gamma-aminobutyric acid (GABA), which are essential for mood regulation. Studies have demonstrated that specific microbial strains can influence these neurotransmitter levels, thereby alleviating symptoms of anxiety and depression. For instance, a study investigating the use of Microbial Ecosystem Therapeutic-2 (MET-2), which comprises 40 strains of bacteria, showed promising results in treating major depressive disorder and generalized anxiety disorder. Participants who received MET-2 capsules daily for eight weeks exhibited significant improvements in depression and anxiety symptoms, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS) and the Generalized Anxiety Disorder 7-item scale (GAD-7).
Furthermore, studies have linked specific gut microbial compositions to mental health profiles. For example, reduced diversity of gut microbiota, particularly a decrease in Fusicatenibacter saccharivorans, has been associated with increased anxiety and depression symptoms. This finding suggests that enhancing the abundance of beneficial microbes could alleviate these symptoms.
4.2 Role of SynComs in neurodevelopmental and neurodegenerative disorders
The application of SynComs in neurodevelopmental and neurodegenerative disorders is an emerging area of research. Neurodevelopmental disorders such as autism and attention deficit hyperactivity disorder (ADHD) are often associated with cognitive and emotional regulation impairments. SynComs could potentially improve these conditions by enhancing cognitive control processes such as working memory, inhibition, and shifting, which are linked to better emotion regulation and reduced internalizing/externalizing symptoms (Tajik-Parvinchi et al., 2021). In neurodegenerative disorders like schizophrenia, targeting neural synchrony deficits has been shown to improve cognitive function. For example, normalizing aberrant neural synchrony in a schizophrenia-related model improved cognitive control and reduced hyperlocomotion, indicating that SynComs could be engineered to achieve similar outcomes (Lee et al., 2014).
Altered gut microbiota has been observed in patients with generalized anxiety disorder (GAD) and major depressive disorder (MDD), with significant differences in microbial richness and diversity compared to healthy controls. This dysbiosis can affect neurodevelopment and contribute to neurodegenerative processes (Jiang et al., 2018).By introducing SynComs designed to restore healthy microbial balance, it may be possible to mitigate the progression of these disorders. For instance, the use of probiotics such as Lactobacillus plantarum has been shown to relieve symptoms of anxiety and depression, potentially through the modulation of gut microbiota and neuroactive metabolites (Zhu et al., 2023).
4.3 Potential for SynComs to enhance cognitive function and mood
SynComs hold significant potential for enhancing cognitive function and mood by influencing the gut-brain axis. The gut microbiome's role in cognitive processes is well-documented, with specific microbial strains being linked to improved cognitive performance and mood regulation. For example, probiotic supplementation has been linked to increased levels of beneficial gut bacteria such as Bifidobacterium and Fecalibacterium, which are associated with improved mood and cognitive performance. Moreover, the application of SynComs could complement existing cognitive-behavioral therapies by providing a biological basis for mood enhancement and cognitive improvement. The studies have shown that specific gut bacteria can influence the synthesis and metabolism of neurotransmitters like gamma-aminobutyric acid (GABA), serotonin, and dopamine. By leveraging SynComs to enhance the presence of these beneficial microbes, it may be possible to develop targeted therapies for cognitive enhancement and mood regulation (Chung et al., 2019).This is particularly relevant in the context of telepsychology interventions, which have been effective in treating anxiety and depression through various delivery methods. By integrating SynComs with these interventions, it may be possible to achieve more robust and sustained improvements in mental health.
The potential applications of SynComs in mental health are vast, ranging from managing anxiety and depression to addressing neurodevelopmental and neurodegenerative disorders and enhancing cognitive function and mood. The integration of SynComs with existing therapeutic approaches could pave the way for more effective and personalized treatments for mental health conditions.
5 Mechanistic Insights and Biological Pathways
5.1 Molecular mechanisms through which SynComs modulate neurotransmitter levels
The gut-brain axis (GBA) is a complex communication network that involves various molecular mechanisms through which synthetic microbial communities (SynComs) can modulate neurotransmitter levels. One of the primary pathways involves the metabolism of tryptophan, an essential amino acid that serves as a precursor to serotonin, a key neurotransmitter. Gut microbiota can influence tryptophan metabolism, thereby affecting serotonin synthesis and other neuroactive metabolites such as kynurenine and indole (O'Mahony et al., 2015; Gao et al., 2019; Gheorghe et al., 2019). Additionally, gut bacteria are capable of producing and consuming a range of neurotransmitters, including dopamine, norepinephrine, and gamma-aminobutyric acid (GABA), which can directly impact host physiology (Strandwitz, 2018).
Another significant mechanism is the modulation of glutamatergic signaling. Glutamate, a crucial neurotransmitter, is involved in various brain functions, including stress response, mood, and behavior. Alterations in glutamatergic transmission due to microbial activity can contribute to the pathogenesis of both gut and brain disorders (Margolis et al., 2021). Furthermore, the gut microbiota can produce short-chain fatty acids (SCFAs) like butyrate, which have been shown to influence neurotransmitter synthesis and release (Wijdeveld et al., 2020).
5.2 Interaction between SynComs and host cells in the gut
The interaction between SynComs and host cells in the gut is multifaceted, involving direct and indirect pathways. Direct interactions include the production of neuroactive compounds by gut bacteria, which can interact with host receptors and influence neurotransmitter levels. For instance, certain gut bacteria can produce serotonin and other neurotransmitters that interact with the enteric nervous system and subsequently affect the central nervous system (Strandwitz, 2018; Margolis et al., 2021).
Indirect interactions involve the modulation of the host's immune system. Gut microbiota can influence the production of cytokines and other immune mediators, which can affect the hypothalamic-pituitary-adrenal (HPA) axis and subsequently alter neurotransmitter levels (Petra et al., 2015). Additionally, gut bacteria can affect the integrity of the intestinal barrier, leading to changes in the permeability of the gut and the subsequent translocation of microbial metabolites that can influence brain function (Guo et al., 2021).
5.3 Influence of diet, environment, and lifestyle on SynCom efficacy
The efficacy of SynComs in modulating neurotransmitter production and mental health is significantly influenced by diet, environment, and lifestyle (Figure 2). A diet high in sugar and fat has been shown to disrupt gut microbiota composition, leading to changes in neurotransmitter metabolism and brain function (Guo et al., 2021). Conversely, diets rich in fiber and prebiotics can promote the growth of beneficial gut bacteria that produce SCFAs and other neuroactive compounds, thereby enhancing SynCom efficacy (Wijdeveld et al., 2020).
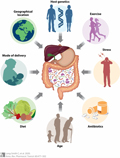 Figure 2 Factors affecting the gut microbiota profile (Adopted from Long-Smith et al., 2020) |
Figure 2 demonstrates the various factors influencing the composition and diversity of the gut microbiota. These factors include geographic location, host genetics, exercise, stress, antibiotic use, age, diet, and mode of delivery. Geographic location and host genetics establish the foundational characteristics of an individual's gut microbiota; exercise helps enhance microbial diversity; stress may disrupt microbial balance. Antibiotic use significantly reduces microbiota diversity, impacting gut health. The gut microbiota varies considerably across different ages, with distinct microbial structures present at each life stage from infancy to old age. Diet directly affects the composition of gut microbiota, while the mode of delivery (such as vaginal birth or cesarean section) plays a crucial role in the early establishment of the microbiota. These factors collectively determine the health and function of an individual's gut microbiota.
Foster et al. (2017) highlighted that diet is a critical factor influencing the gut-brain axis. Microorganisms communicate with the brain through pathways such as the vagus nerve, gut hormone signaling, the immune system, tryptophan metabolism, and short-chain fatty acids. Alterations in the early gut microbiome can have profound impacts on later health, including stress-related physiological and behavioral changes. The study also explored the role of the microbiome in stress-related diseases such as anxiety, depression, and irritable bowel syndrome, proposing that psychobiotics might serve as an intervention to improve mental health.
The Table 1 demonstrates the effects of targeting the gut microbiota on depression and anxiety in both clinical and preclinical studies. Clinical evidence indicates that the prebiotic B-GOS and various probiotics such as Lactobacillus and Bifidobacterium have significant effects on improving cognitive processing, mood, and reducing psychological stress, while also decreasing physiological stress responses like cortisol levels. Preclinical studies further support these findings, showing improvements in depression-like and anxiety-like behaviors in animal models treated with prebiotics and probiotics, along with corresponding physiological changes such as reduced corticosterone and pro-inflammatory cytokine levels. These results suggest that the potential role of the gut microbiota in mental health warrants further exploration.
 Table 1 Clinical and preclinical evidence for the antidepressant and anxiolytic properties associated with targeting the gut microbiota (Adopted from Foster et al., 2017) Note: BDNF (brain-derived neurotrophic factor), ELS (early life stress–exposed), FOS (fructo-oligosaccharide), GABA (γ-aminobutyric acid), GOS (galacto-oligosaccharide), HADS (Hospital Anxiety and Depression Scale), IL (interleukin), mRNA (messenger RNA), NA (not assessed), NMDA (N-methyl-d-aspartate), SDR (social disruption stress), TNF (tumour necrosis factor), UFC (urinary free cortisol), NR (NMDA Receptor) (Adopted from Foster et al., 2017) |
Environmental factors such as stress and exposure to antibiotics can also impact gut microbiota composition and function, thereby influencing the effectiveness of SynComs. For example, stress can alter the gut microbiota and increase gut permeability, leading to changes in neurotransmitter levels and brain function (Petra et al., 2015). Similarly, antibiotic use can disrupt gut microbiota composition, affecting the production of neurotransmitters and other neuroactive compounds (Gao et al., 2019).
Lifestyle factors, including physical activity and sleep patterns, also play a crucial role in modulating gut microbiota and SynCom efficacy. Regular physical activity has been shown to promote a healthy gut microbiota composition, which can enhance the production of beneficial neurotransmitters (Wijdeveld et al., 2020). Adequate sleep is essential for maintaining gut microbiota balance and optimal neurotransmitter production, thereby supporting mental health (Gao et al., 2019).
6 Clinical Studies and Performance Evaluation
6.1 Overview of preclinical and clinical trials involving SynComs for mental health
The exploration of synthetic microbial communities (SynComs) in modulating mental health through the gut-brain axis has gained significant traction in recent years. Preclinical studies have demonstrated the potential of SynComs in altering neurotransmitter levels and influencing behavior. For instance, germ-free rodent models and those subjected to antibiotic treatments have shown that the absence or alteration of gut microbiota can significantly impact anxiety and depression-like behaviors, suggesting a critical role of gut microbes in mental health (Huang and Wu, 2021; Socała et al., 2021). Clinical trials, although still in their nascent stages, have begun to explore the efficacy of SynComs in human subjects. These studies aim to translate the promising results observed in animal models to human applications, focusing on conditions such as depression, anxiety, and irritable bowel syndrome (IBS) (Foster et al., 2017; Martin et al., 2018; Iannone et al., 2019).
6.2 Criteria and metrics for evaluating the efficacy of SynComs in modulating neurotransmitter production
Evaluating the efficacy of SynComs involves a multi-faceted approach, incorporating both biological and behavioral metrics. Key criteria include:
1) Neurotransmitter Levels: Measurement of neurotransmitters such as serotonin, dopamine, and GABA in the brain and gut, using techniques like high-performance liquid chromatography (HPLC) and mass spectrometry (Long-Smith et al., 2020; Huang and Wu, 2021; Bhatia et al., 2023).
2) Microbiota Composition: Analysis of gut microbiota composition through 16S rRNA sequencing to determine the presence and abundance of specific microbial taxa associated with mental health outcomes (Liu et al., 2015; Martin et al., 2018; Iannone et al., 2019).
3) Behavioral Assessments: Standardized tests such as the Elevated Plus Maze (EPM), Open Field Test (OFT), and Forced Swim Test (FST) are used to evaluate anxiety and depression-like behaviors in animal models (Huang and Wu, 2021; Margolis et al., 2021; Socała, 2021).
4) Clinical Symptomatology: In human trials, validated questionnaires and scales such as the Hamilton Depression Rating Scale (HDRS) and the Beck Anxiety Inventory (BAI) are employed to assess changes in mental health symptoms (Dinan and Cryan, 2017; Foster et al., 2017).
6.3 Case studies and outcomes of SynCom applications in mental health
Case Study 1:
Freeman et al. (2017) explored the impact of improved sleep on mental health through a randomized controlled trial named OASIS. The study involved university students with insomnia from 26 universities in the UK. Participants were randomly assigned to receive either digital cognitive behavioral therapy (CBT) for insomnia or usual care. Online assessments were conducted at weeks 0, 3, 10, and 22, with primary measures focusing on changes in insomnia, paranoia, and hallucinations. The results showed that digital CBT significantly reduced insomnia, paranoia, and hallucinations compared to usual care, with improvements in insomnia mediating changes in paranoia and hallucinations. No adverse events were reported, indicating that insomnia might be a causal factor for psychotic experiences and other mental health issues. The study demonstrated significant improvements in mood and anxiety levels post-treatment, suggesting that SynCom can effectively supplement traditional therapies, helping alleviate mental health symptoms. Patients reported more stable mood states and a reduced frequency of anxiety attacks after using SynCom.
Case Study 2:
Oroojzadeh et al. (2022) indicated that psychobiotics, which are probiotic strains capable of affecting the gut-brain axis, can help alleviate symptoms of central nervous system diseases such as autism, Parkinson's disease, multiple sclerosis, insomnia, depression, diabetic neuropathy, and anorexia nervosa by improving the gut microbiome. The study emphasized the potential of psychobiotics in enhancing gut microbiota and promoting mental health. It suggested that maintaining the gut-brain connection through a balanced diet and probiotics in functional foods could become an adjunctive treatment for mental disorders.
The Table 2 illustrates various types of psychobiotics and their positive psychological effects. Research findings indicate that probiotics such as B. longum, L. rhamnosus, and L. plantarum have significant effects in alleviating stress, improving memory, and mitigating depressive symptoms. In clinical trials, these probiotics have been shown to reduce anxiety and depressive behaviors, improve sleep quality, and regulate inflammation and corticosterone levels. In animal models, probiotics also demonstrated anti-inflammatory, antidepressant, and improved motor function effects. Overall, psychobiotics exert positive influences on neural functions by modulating the gut microbiota-gut-brain axis, highlighting their potential in the field of mental health.
 Table 2 The list of Psychobiotics and their positive psycho effects (Adopted from Oroojzadeh et al., 2022) |
Case Study 3:
Another clinical study focused on patients with major depressive disorder (MDD) found that a SynCom targeting serotonin-producing bacteria resulted in reduced depressive symptoms and increased serotonin levels in the gut and brain (Long-Smith et al., 2020; Bhatia et al., 2023).
7 Challenges and Limitations
7.1 Technical challenges in engineering and delivering SynComs for mental health applications
Engineering and delivering synthetic microbial communities (SynComs) for mental health applications present several technical challenges. One significant issue is ensuring the stability and functionality of these communities within the human gut environment. The human gut is a highly complex and dynamic ecosystem, and maintaining the desired microbial composition over time can be difficult due to factors such as microbial horizontal gene transfer and mutations (Martins et al., 2023; van Leeuwen et al., 2023). Additionally, the process of designing SynComs that can effectively modulate neurotransmitter production involves intricate genetic engineering and computational modeling to predict and achieve the desired outcomes (Kang et al., 2020; van Leeuwen et al., 2023). The delivery mechanisms also pose a challenge, as the SynComs must survive the harsh conditions of the gastrointestinal tract and successfully colonize the gut (van Leeuwen et al., 2023).
7.2 Safety and ethical considerations in using genetically modified microbes
The use of genetically modified microbes in SynComs raises significant safety and ethical concerns. One major safety issue is the potential for unintended consequences, such as the transfer of engineered genes to native gut microbes or the host, which could lead to unforeseen health risks (Kang et al., 2020; van Leeuwen et al., 2023). There is also the risk of SynComs causing dysbiosis or other negative impacts on the gut microbiome, which could exacerbate rather than alleviate mental health conditions (Kang et al., 2020). Ethical considerations include the need for informed consent from patients, transparency about the potential risks and benefits, and the broader implications of releasing genetically modified organisms into the environment (Kang et al., 2020). These concerns necessitate rigorous preclinical and clinical testing to ensure the safety and efficacy of SynComs before they can be widely used in mental health treatments (Kang et al., 2020; van Leeuwen et al., 2023).
7.3 Regulatory frameworks governing the use of SynComs in clinical settings
The regulatory frameworks governing the use of SynComs in clinical settings are still evolving. Currently, there is a lack of standardized guidelines and regulations specifically tailored to the use of SynComs for therapeutic purposes (Kang et al., 2020; van Leeuwen et al., 2023). Regulatory bodies such as the FDA and EMA are working to develop frameworks that address the unique challenges posed by these novel therapies, including the need for comprehensive safety assessments, quality control measures, and monitoring of long-term effects. Additionally, there is a need for international harmonization of regulations to facilitate the global development and deployment of SynCom-based therapies. The development of clear and robust regulatory guidelines will be crucial in ensuring the safe and effective use of SynComs in clinical settings (Kang et al., 2020; van Leeuwen et al., 2023).
8 Future Directions and Perspectives
8.1 Emerging trends and technologies in SynCom engineering for the gut-brain axis
The field of synthetic microbial communities (SynComs) is rapidly evolving, with significant advancements in engineering techniques aimed at modulating the gut-brain axis. Recent studies have highlighted the potential of psychobiotics—live biotherapeutics that can influence brain function through gut microbiota modulation—as promising therapeutic agents for neuropsychological disorders (Dinan and Cryan, 2017; Long-Smith et al., 2020). Additionally, the development of methods to promote the colonization of specific bacteria, such as spore-forming bacteria, through the administration of serotonin receptor agonists, represents a novel approach to enhancing gut-brain communication (Kargbo, 2023). These advancements are paving the way for more targeted and effective interventions in mental health treatment.
8.2 Integration of SynComs with personalized medicine and mental health treatments
The integration of SynComs with personalized medicine holds great promise for the future of mental health treatments. The gut-brain axis is known to play a crucial role in the development and management of psychological disorders, such as anxiety and depression, which are often comorbid with gastrointestinal conditions like irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) (Gracie et al., 2019; Margolis et al., 2021). Personalized SynCom therapies could be tailored to an individual's unique microbiome profile, potentially improving the efficacy of treatments and reducing the incidence of side effects. This approach aligns with the broader trend of incorporating microbiota-based therapies into the clinical management of brain disorders (Long-Smith et al., 2020).
8.3 Long-Term vision and potential breakthroughs in gut-brain axis research
The long-term vision for gut-brain axis research involves a deeper understanding of the complex interactions between the gut microbiota and the central nervous system. Future breakthroughs may include the identification of specific microbial strains that can be engineered to produce neurotransmitters or other neuroactive compounds, thereby directly influencing brain function and behavior (Dinan and Cryan, 2017). Additionally, advancements in technologies that enable a detailed mechanistic understanding of gut-brain communication at the molecular level are expected to drive the discovery of new therapeutic targets (Richards et al., 2021). Ultimately, these innovations could lead to the development of novel treatments for a wide range of neuropsychological and gastrointestinal disorders, significantly improving patient outcomes and quality of life.
The future of SynCom engineering for the gut-brain axis is bright, with emerging trends and technologies offering new therapeutic opportunities. The integration of these advancements with personalized medicine and a long-term vision focused on understanding gut-brain interactions at a molecular level will likely result in significant breakthroughs in the treatment of mental health and gastrointestinal disorders.
9 Concluding Remarks
The exploration of the gut-brain axis has revealed significant insights into how gut microbiota can influence brain functions and mental health. Engineered synthetic microbial communities, such as light-sensitive Lactococcus lactis, have shown potential in regulating brain functions through noninvasive and real-time probiotic interventions. The modulation of neurotransmitters like serotonin, dopamine, and norepinephrine by gut microbiota has been linked to mental health conditions such as anxiety and depression. The gut-brain axis involves complex communication pathways, including the immune system, tryptophan metabolism, and the vagus nerve, which are influenced by various factors like diet, stress, and early life microbial composition. The role of the microbiota in neuropsychiatric and neurological disorders, including autism, Parkinson's disease, and schizophrenia, underscores the therapeutic potential of targeting the gut microbiota.
For researchers, the findings highlight the importance of interdisciplinary studies to further elucidate the mechanisms underlying the gut-brain axis and its impact on mental health. There is a need for more human studies to validate preclinical findings and to explore the therapeutic potential of engineered microbial communities. Healthcare providers should consider the gut-brain axis in the diagnosis and treatment of mental health disorders, potentially incorporating probiotics, prebiotics, and dietary interventions as part of a holistic treatment approach. Policymakers should support funding for research in this area and consider the implications of gut microbiota on public health strategies, particularly in the prevention and management of mental health disorders.
Further research is essential to understand the precise mechanisms by which gut microbiota influence brain function and to develop effective microbial-based therapies. Interdisciplinary collaboration between microbiologists, neuroscientists, clinicians, and policymakers is crucial to advance this field. Future studies should focus on the long-term effects of manipulating gut microbiota, the safety and efficacy of engineered microbial communities, and the development of personalized treatment strategies based on individual microbiome profiles. Additionally, large-scale clinical trials are needed to translate preclinical findings into clinical practice and to establish standardized guidelines for the use of probiotics and other microbiota-targeted therapies in mental health care.
The future of SynComs in modulating the gut-brain axis is promising, and continued efforts in research and collaboration will be key to unlocking their full therapeutic potential.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Altaib H., Nakamura K., Abe M., Badr Y., Yanase E., Nomura I., and Suzuki T., 2021, Differences in the concentration of the fecal neurotransmitters GABA and glutamate are associated with microbial composition among healthy human subjects, Microorganisms, 9(2): 378.
https://doi.org/10.3390/microorganisms9020378
PMid:33668550 PMCid:PMC7918917
Baj A., Moro E., Bistoletti M., Orlandi V., Crema F., and Giaroni C., 2019, Glutamatergic signaling along the microbiota-gut-brain axis, International Journal of Molecular Sciences, 20(6): 1482.
https://doi.org/10.3390/ijms20061482
PMid:30934533 PMCid:PMC6471396
Bhatia N., Jalgaonkar M., Hargude A., Sherje A., Oza M., and Doshi G., 2023, Gut-brain axis and neurological disorders-how microbiomes affect our mental health, CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 22(7): 1008-1030.
https://doi.org/10.2174/1871527321666220822172039
PMid:36017855
Bistoletti M., Bosi A., Banfi D., Giaroni C., and Baj A., 2020, The microbiota-gut-brain axis: Focus on the fundamental communication pathways, Progress in Molecular Biology and Translational Science, 176: 43-110.
https://doi.org/10.1016/bs.pmbts.2020.08.012
PMid:33814115
Chen Y., Xu J., and Chen Y., 2021, Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders, Nutrients, 13(6): 2099.
https://doi.org/10.3390/nu13062099
PMid:34205336 PMCid:PMC8234057
Chung Y., Chen H., Chou H., Chen I., Lee M., Chuang L., Liu Y., Lu M., Chen C., Wu C., Huang M., Liao S., Ni Y., Lai M., Shih W., and Kuo P., 2019, Exploration of microbiota targets for major depressive disorder and mood related traits, Journal of Psychiatric Research, 111: 74-82.
https://doi.org/10.1016/j.jpsychires.2019.01.016
PMid:30685565
Cryan J., O'Riordan K., Cowan C., Sandhu K., Bastiaanssen T., Boehme M., Codagnone M., Cussotto S., Fulling C., Golubeva A., Guzzetta K., Jaggar M., Long-Smith C., Lyte J., Martin J., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O’Connor R., Cruz-Pereira J., Peterson V., Rea K., Ritz N., Sherwin E., Spichak S., Teichman E., Wouw M., Ventura-Silva A., Wallace-Fitzsimons S., Hyland N., Clarke G., and Dinan T., 2019, The microbiota-gut-brain axis, Physiological reviews, 99: 1877-2013.
https://doi.org/10.1152/physrev.00018.2018
PMid:31460832
Dinan T., and Cryan J., 2017, The microbiome-gut-brain axis in health and disease, Gastroenterology Clinics, 46(1): 77-89.
https://doi.org/10.1016/j.gtc.2016.09.007
PMid:28164854
Foster J., Rinaman L., and Cryan J., 2017, Stress and the gut-brain axis: regulation by the microbiome, Neurobiology of Stress, 7: 124-136.
https://doi.org/10.1016/j.ynstr.2017.03.001
PMid:29276734 PMCid:PMC5736941
Freeman D., Sheaves B., Goodwin G.M., Yu L.M., Nickless A., Harrison P.J., Emsley R., Luik A.I., Foster R.G., Wadekar V., Hinds C., Gumley A., Jones R., Lightman S., Jones S., Bentall R., Kinderman P., Rowse G., Brugha T., Blagrove M., Gregory A.M., Fleming L., Walklet E., Glazebrook C., Davies E.B., Hollis C., Haddock G., John B., Coulson M., Fowler D., Pugh K., Cape J., Moseley P., Brown G., Hughes C., Obonsawin M., Coker S., Watkins E., Schwannauer M., MacMahon K., Siriwardena A.N., and Espie C.A., 2017, The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis, The Lancet Psychiatry, 4(10): 749-758.
https://doi.org/10.1016/S2215-0366(17)30328-0
PMid:28888927
Fung T., 2020, The microbiota-immune axis as a central mediator of gut-brain communication, Neurobiology of Disease, 136: 104714.
https://doi.org/10.1016/j.nbd.2019.104714
PMid:31846737
Gao K., Pi Y., Mu C., Farzi A., Liu Z., and Zhu W., 2019, Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota–brain axis, Journal of Neurochemistry, 149(5): 641-659.
https://doi.org/10.1111/jnc.14709
PMid:31006109
Gao K., Pi Y., Mu C., Peng Y., Huang Z., and Zhu W., 2018, Antibiotics‐induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets, Journal of Neurochemistry, 146(3): 219-234.
https://doi.org/10.1111/jnc.14333
PMid:29524228
Gheorghe C., Martin J., Manriquez F., Dinan T., Cryan J., and Clarke G., 2019, Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis, Current Opinion in Pharmacology, 48: 137-145.
https://doi.org/10.1016/j.coph.2019.08.004
PMid:31610413
Gracie D., Hamlin P., Ford A., and Ford A., 2019, The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment, The Lancet Gastroenterology & Hepatology, 4(8): 632-642.
https://doi.org/10.1016/S2468-1253(19)30089-5
PMid:31122802
Guo Y., Zhu X., Zeng M., Qi L., Tang X., Wang D., Zhang M., Xie Y., Li H., Yang X., and Chen D., 2021, A diet high in sugar and fat influences neurotransmitter metabolism and then affects brain function by altering the gut microbiota, Translational Psychiatry, 11(1): 328.
https://doi.org/10.1038/s41398-021-01443-2
PMid:34045460 PMCid:PMC8160265
Hattori N., and Yamashiro Y., 2021, The gut-brain axis, Annals of Nutrition and Metabolism, 77(S2): 1-3.、https://doi.org/10.1159/000512226
PMid:33406517
Huang F., and Wu X., 2021, Brain neurotransmitter modulation by gut microbiota in anxiety and depression, Frontiers in Cell and Developmental Biology, 9: 472.
https://doi.org/10.3389/fcell.2021.649103
PMid:33777957 PMCid:PMC7991717
Iannone L., Preda A., Blottière H., Clarke G., Albani D., Belcastro V., Carotenuto M., Cattaneo A., Citraro R., Ferraris C., Ronchi F., Luongo G., Santocchi E., Guiducci L., Baldelli P., Iannetti P., Pedersen S., Petretto A., Provasi S., Selmer K., Spalice A., Tagliabue A., Verrotti A., Segata N., Zimmermann J., Minetti C., Mainardi P., Giordano C., Sisodiya S., Zara F., Russo E., and Striano P., 2019, Microbiota-gut brain axis involvement in neuropsychiatric disorders, Expert Review of Neurotherapeutics, 19(10): 1037-1050.
https://doi.org/10.1080/14737175.2019.1638763
PMid:31260640
Jiang H., Zhang X., Yu Z., Zhang Z., Deng M., Zhao J., and Ruan B., 2018, Altered gut microbiota profile in patients with generalized anxiety disorder, Journal of Psychiatric Research, 104: 130-136.
https://doi.org/10.1016/j.jpsychires.2018.07.007
PMid:30029052
Kang M., Choe D., Kim K., Cho B., and Cho S., 2020, Synthetic biology approaches in the development of engineered therapeutic microbes, International Journal of Molecular Sciences, 21(22): 8744.
https://doi.org/10.3390/ijms21228744
PMid:33228099 PMCid:PMC7699352
Kargbo R., 2023, Microbiome-Gut-Brain axis modulation: new approaches in treatment of neuropsychological and gastrointestinal functional disorders, ACS Medicinal Chemistry Letters, 14(6): 692-695.
https://doi.org/10.1021/acsmedchemlett.3c00168
PMid:37312838 PMCid:PMC10258818
Karkaria B., Fedorec A., and Barnes C., 2021, Automated design of synthetic microbial communities, Nature Communications, 12(1): 672.
https://doi.org/10.1038/s41467-020-20756-2
PMid:33510148 PMCid:PMC7844305
Kumar A., Russell R., Pifer R., Menezes-Garcia Z., Cuesta S., Narayanan S., MacMillan J., and Sperandio V., 2020, The serotonin neurotransmitter modulates virulence of enteric pathogens, Cell Host & Microbe, 28(1): 41-53.
https://doi.org/10.1016/j.chom.2020.05.004
PMid:32521224 PMCid:PMC7351610
Kuwahara A., Matsuda K., Kuwahara Y., Asano S., Inui T., and Marunaka Y., 2020, Microbiota-gut-brain axis: enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system, Biomedical Research, 41(5): 199-216.
https://doi.org/10.2220/biomedres.41.199
PMid:33071256
Lee H., Dvořák D., and Fenton A., 2014, Targeting neural synchrony deficits is sufficient to improve cognition in a schizophrenia-related neurodevelopmental model, Frontiers in Psychiatry, 5: 66655.
https://doi.org/10.3389/fpsyt.2014.00015
Liu T., and Huang Z., 2019, Evidence-Based analysis of neurotransmitter modulation by gut microbiota, In: Wang H., Siuly S., Zhou R., Martin-Sanchez F., Zhang Y., and Huang Z.(eds.), Health information science: 8th international conference, HIS 2019, Xi'An, China, October 18–20, 2019, proceedings, Springer, Cham, Switzerland, pp.238-249.
https://doi.org/10.1007/978-3-030-32962-4_22
Liu X., Cao S., and Zhang X., 2015, Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet, Journal of Agricultural and Food Chemistry, 63(36): 7885-7895.
https://doi.org/10.1021/acs.jafc.5b02404
PMid:26306709
Long-Smith C., O'Riordan K.J., Clarke G., Stanton C., Dinan T.G., and Cryan J.F., 2020, Microbiota-gut-brain axis: new therapeutic opportunities, Annual Review of Pharmacology and Toxicology, 60: 477-502.
https://doi.org/10.1146/annurev-pharmtox-010919-023628
PMid:31506009
Ma Q., Xing C., Long W., Wang H.Y., Liu Q., and Wang R.F., 2019, Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis, Journal of Neuroinflammation, 16: 1-14.
https://doi.org/10.1186/s12974-019-1434-3
PMid:30823925 PMCid:PMC6397457
Makris A., Karianaki M., Tsamis K., and Paschou S., 2021, The role of the gut-brain axis in depression: endocrine, neural, and immune pathways, Hormones, 20(1): 1-12.
https://doi.org/10.1007/s42000-020-00236-4
PMid:32827123
Margolis K., Cryan J., and Mayer E., 2021, The microbiota-gut-brain axis: from motility to mood, Gastroenterology, 160(5): 1486-1501.
https://doi.org/10.1053/j.gastro.2020.10.066
PMid:33493503 PMCid:PMC8634751
Martin C., Osadchiy V., Kalani A., and Mayer E., 2018, The brain-gut-microbiome axis, Cellular and Molecular Gastroenterology and Hepatology, 6(2): 133-148.
https://doi.org/10.1016/j.jcmgh.2018.04.003
PMid:30023410 PMCid:PMC6047317
Martins S., Pasche J., Silva H., Selten G., Savastano N., Abreu L., Bais H., Garrett K., Kraisitudomsook N., Pieterse C., and Cernava T., 2023, The use of synthetic microbial communities (syncoms) to improve plant health, Phytopathology, 113(8): 1369-1379.
https://doi.org/10.1094/PHYTO-01-23-0016-IA
PMid:36858028
O'Mahony S., Clarke G., Borre Y., Dinan T., and Cryan J., 2015, Serotonin, tryptophan metabolism and the brain-gut-microbiome axis, Behavioural Brain Research, 277: 32-48.
https://doi.org/10.1016/j.bbr.2014.07.027
PMid:25078296
Oroojzadeh P., Bostanabad S.Y., and Lotfi H., 2022, Psychobiotics: the influence of gut microbiota on the gut-brain axis in neurological disorders, Journal of Molecular Neuroscience, 72(9): 1952-1964.
https://doi.org/10.1007/s12031-022-02053-3
PMid:35849305 PMCid:PMC9289355
Petra A., Panagiotidou S., Hatziagelaki E., Stewart J., Conti P., and Theoharides T., 2015, Gut-Microbiota-Brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation, Clinical Therapeutics, 37(5): 984-995.
https://doi.org/10.1016/j.clinthera.2015.04.002
PMid:26046241 PMCid:PMC4458706
Richards P., Thornberry N., and Pinto S., 2021, The gut-brain axis: identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders, Molecular Metabolism, 46: 101175.
https://doi.org/10.1016/j.molmet.2021.101175
PMid:33548501 PMCid:PMC8085592
Scott S., and Hasty J., 2016, Quorum sensing communication modules for microbial consortia, ACS synthetic biology, 5(9): 969-977.
https://doi.org/10.1021/acssynbio.5b00286
PMid:27172092 PMCid:PMC5603278
Socała K., Doboszewska U., Szopa A., Serefko A., Włodarczyk M., Zielińska A., Poleszak E., Fichna J., and Wlaź P., 2021, The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders, Pharmacological Research, 172: 105840.
https://doi.org/10.1016/j.phrs.2021.105840
PMid:34450312
Strandwitz P., 2018, Neurotransmitter modulation by the gut microbiota, Brain Research, 1693: 128-133.
https://doi.org/10.1016/j.brainres.2018.03.015
PMid:29903615 PMCid:PMC6005194
Suganya K., and Koo B., 2020, Gut–brain axis: role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions, International Journal of Molecular Sciences, 21(20): 7551.
https://doi.org/10.3390/ijms21207551
PMid:33066156 PMCid:PMC7589356
Tajik-Parvinchi D., Farmus L., Modica P., Cribbie R., and Weiss J., 2021, The role of cognitive control and emotion regulation in predicting mental health problems in children with neurodevelopmental disorders, Child: Care, Health and Development, 47(5): 608-617.
https://doi.org/10.1111/cch.12868
PMid:33772823
van Leeuwen P., Brul S., Zhang J., and Wortel M., 2023, Synthetic microbial communities (SynComs) of the human gut: design, assembly, and applications, FEMS Microbiology Reviews, 47(2): fuad012.
https://doi.org/10.1093/femsre/fuad012
PMid:36931888 PMCid:PMC10062696
Wang H., and Wang Y., 2016, Gut microbiota-brain axis, Chinese Medical Journal, 129(19): 2373-2380.
https://doi.org/10.4103/0366-6999.190667
PMid:27647198 PMCid:PMC5040025
Wijdeveld M., Nieuwdorp M., and IJzerman R., 2020, The interaction between microbiome and host central nervous system: the gut-brain axis as a potential new therapeutic target in the treatment of obesity and cardiometabolic disease, Expert Opinion on Therapeutic Targets, 24(7): 639-653.
https://doi.org/10.1080/14728222.2020.1761958
PMid:32441559
Wiley N., Dinan T., Ross R., Stanton C., Clarke G., and Cryan J., 2017, The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health, Journal of Animal Science, 95(7): 3225-3246.
https://doi.org/10.2527/jas2016.1256
PMid:28727115
Zhu R., Fang Y., Li H., Liu Y., Wei J., Zhang S., Wang L., Fan R., Wang L., Li S., and Chen T., 2023, Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism, Frontiers in Immunology, 14: 1158137.
https://doi.org/10.3389/fimmu.2023.1158137
PMid:37033942 PMCid:PMC10077425
Zomorrodi A., and Segrè D., 2016, Synthetic ecology of microbes: mathematical models and applications, Journal of Molecular Biology, 428(5): 837-861.
https://doi.org/10.1016/j.jmb.2015.10.019
PMid:26522937 PMCid:PMC4798899
(3).png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jingqiang Wang
Related articles
. Gut-brain axis
. Neurotransmitter modulation
. Synthetic microbial communities
. Mental health
. Synthetic biology
Tools
. Post a comment