Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 4
Received: 08 Jul., 2024 Accepted: 10 Aug., 2024 Published: 23 Aug., 2024
Alzheimer's disease (AD) is a progressive neurodegenerative disorder primarily driven by the accumulation of amyloid-β plaques and neurofibrillary tangles. Despite advances in understanding its molecular mechanisms, current treatments remain largely symptomatic. Gene therapy, by targeting specific genetic mutations and pathological pathways, offers a promising approach to addressing the root causes of AD. This study explores the potential of gene therapy in AD, with a focus on the latest advancements in gene editing technologies, such as CRISPR/Cas9, the development of innovative gene delivery systems, and the rise of personalized medicine. The study also discusses the challenges of clinical translation for gene therapy, including technical, ethical, and regulatory hurdles. Finally, the research highlights future directions in gene therapy, particularly the exploration of new therapeutic targets beyond amyloid and tau, and the integration of gene therapy with other treatment modalities to achieve synergistic effects. Despite significant challenges, the prospects of gene therapy in AD offer great hope for fundamentally altering the treatment landscape of this disease.
1 Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that stands as the most common cause of dementia, affecting millions of individuals worldwide. Characterized by cognitive decline, memory loss, and behavioral changes, AD imposes a significant burden not only on patients but also on caregivers and healthcare systems globally. The pathophysiology of AD is complex, involving the accumulation of amyloid-beta (Aβ) plaques, the formation of neurofibrillary tangles composed of hyperphosphorylated tau protein, and widespread neuronal loss. Despite extensive research and the development of various therapeutic strategies, there remains no cure for AD, and current treatments are primarily symptomatic, providing only temporary relief without altering the disease's course (Weber-Adrian, 2019; Owens et al., 2021). The progressive nature of AD and the lack of effective disease-modifying treatments underscore the urgent need for innovative therapeutic approaches (Ghaffari et al., 2020).
Given the limitations of existing therapies, there is a growing interest in novel approaches that target the underlying molecular and genetic mechanisms of AD. Gene therapy, which involves the modification or manipulation of gene expression within a patient's cells, offers a promising avenue for the treatment of AD. Unlike conventional therapies that focus on alleviating symptoms, gene therapy aims to correct or modulate the genetic defects and pathological processes that drive the disease. This approach has the potential to not only slow the progression of AD but also to address the root causes of the disorder (Loera-Valencia et al., 2018; Sudhakar and Richardson, 2018). Advances in gene delivery systems, including viral and non-viral vectors, along with breakthroughs in gene-editing technologies like CRISPR/Cas9, have paved the way for innovative gene therapy strategies that could revolutionize the treatment of AD (Rudenko and Sholomon, 2023; Unnisa et al., 2023).
This study will provide a comprehensive analysis of the current status and future prospects of gene therapy in the treatment of Alzheimer's disease (AD). It will explore the genetic basis of AD, the mechanisms and targets of gene therapy, and the current therapeutic strategies. The study will also discuss ongoing research and clinical trials, addressing the challenges and limitations of gene therapy in this context. By synthesizing the latest research and insights, this study will offer a detailed explanation of the critical role that gene therapy plays in tackling Alzheimer's disease.
2 Genetic Underpinnings of Alzheimer's Disease
2.1 Key genetic mutations in AD
Alzheimer's disease (AD) has been extensively studied in the context of genetic predispositions, particularly with mutations in the APP (Amyloid Precursor Protein), PSEN1 (Presenilin 1), and PSEN2 (Presenilin 2) genes. These mutations are most commonly associated with early-onset familial Alzheimer's disease (EOAD), which follows an autosomal dominant inheritance pattern. APP mutations were among the first to be identified and are known to influence the production of amyloid-beta (Aβ), a peptide that aggregates to form the plaques characteristic of AD. Mutations in PSEN1 are the most common cause of EOAD, accounting for the majority of cases, and are often associated with a more aggressive disease course (Gao et al., 2019). Meanwhile, PSEN2 mutations are rarer and typically result in a later onset compared to PSEN1 mutations, although they still contribute to familial AD (Guven et al., 2021).
The APOE (Apolipoprotein E) gene, particularly the ε4 allele, plays a significant role in sporadic forms of AD, which are more common than familial cases. The presence of the APOE ε4 allele is associated with an increased risk of developing late-onset AD (LOAD) and is considered the strongest genetic risk factor for this form of the disease. Interestingly, in the context of autosomal dominant AD, the APOE ε4 allele also influences disease onset and severity, particularly in those carrying APP and PSEN1 mutations, highlighting the complex interplay between these genetic factors (Almkvist and Graff, 2021).
2.2 Pathophysiology and genetic influence
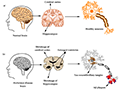
The mutations in APP, PSEN1, and PSEN2 genes primarily influence the production and processing of amyloid-beta, leading to its accumulation in the brain, a hallmark of AD. APP mutations typically increase the production of the amyloidogenic Aβ42 peptide, which is prone to aggregation. Mutations in PSEN1 and PSEN2 are known to affect the gamma-secretase complex, which plays a crucial role in the cleavage of APP to produce Aβ42. This dysregulation leads to an increased ratio of Aβ42 to Aβ40, further promoting plaque formation and neurodegeneration (Figure 1) (Costa-Laparra et al., 2023).
|
Figure 1 The physiological structure of the brain and neurons in (a) healthy brain and (b) Alzheimer’s disease (AD) brain (Adopted from Breijyeh and Karaman, 2020) |
Genetic heterogeneity in AD has significant implications for treatment strategies. The variability in mutations, even within the same gene, can lead to differences in disease onset, progression, and response to treatment. For instance, different PSEN1 mutations can result in varying clinical phenotypes, which necessitates personalized approaches to therapy (Shim et al., 2022). Understanding these genetic differences is critical in developing targeted interventions that can more effectively address the specific pathogenic mechanisms at play in each individual.
2.3 Environmental and lifestyle interactions
In addition to genetic mutations, environmental factors and lifestyle choices play a significant role in the progression of AD, particularly in individuals with a genetic predisposition. Gene-environment interactions are crucial in modulating the onset and severity of the disease. For example, carriers of the APOE ε4 allele are more susceptible to environmental risk factors such as poor diet, lack of physical activity, and exposure to toxins, which can exacerbate the pathogenic processes leading to AD (Lacour et al., 2019).
Moreover, lifestyle interventions that target these modifiable risk factors, such as maintaining a healthy diet, regular exercise, and cognitive stimulation, have been shown to mitigate the effects of genetic risks. These findings highlight the potential for gene-environment interactions to be leveraged in preventive strategies aimed at reducing the incidence and delaying the onset of AD, particularly in genetically at-risk populations (Giau et al., 2019).
3 Mechanisms and Targets of Gene Therapy in AD
3.1 Amyloid-beta and tau pathways
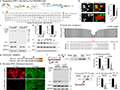
Gene therapy approaches targeting the amyloid-beta (Aβ) plaques and tau tangles, the two main pathological hallmarks of Alzheimer's disease (AD), have shown promise in mitigating disease progression. The accumulation of Aβ peptides, resulting from the cleavage of amyloid precursor protein (APP) by β- and γ-secretases, triggers a cascade leading to tau hyperphosphorylation and aggregation into neurofibrillary tangles (Roda et al., 2022). Gene therapy strategies focus on either reducing the production of Aβ or enhancing its clearance. For instance, targeting the BACE1 enzyme, which is involved in Aβ production, or using gene-editing tools like CRISPR/Cas9 to correct mutations in APP, PSEN1, and PSEN2 genes, has been explored to reduce amyloid plaque formation (Figure 2) (Sun et al., 2018). Additionally, tau-targeting therapies, such as using RNA interference or CRISPR/Cas9 to prevent tau hyperphosphorylation, are being developed to reduce neurofibrillary tangle formation and subsequent neuronal damage (Kent et al., 2020).
|
Figure 2 Strategy and manipulation of the amyloid pathway by CRISPR/Cas9 editing (Adopted from Sun et al., 2018) Image caption: The study utilized CRISPR/Cas9 technology to target the C-terminal of the amyloid precursor protein (APP), which is associated with Alzheimer's disease, and successfully achieved gene editing; a: This panel shows the sgRNA target sequence and its location within the genome, with antibody Y188 used to confirm successful editing of the APP C-terminal; b: This panel illustrates that the fluorescence signal of APP in neuroblastoma cells treated with CRISPR/Cas9 is significantly reduced, indicating successful editing of APP; c and d: These panels further confirm, using immunoblotting, the reduction of the APP C-terminal fragment (APP-CTF) following CRISPR treatment, while the N-terminal signal remains unchanged, indicating that the editing specifically affects the APP C-terminal; e: This panel shows the time course of the editing effect, with a gradual decrease in APP-CTF levels after editing; f: This panel demonstrates the efficient editing of mouse APP at the expected target site, validated by deep genome sequencing; g: This panel compares the APP target sequences in humans and mice, noting minor differences but showing that effective editing can still be achieved using human-specific sgRNA; h-i: These panels show that APP C-terminal editing was also successfully achieved in human iPSC-derived neurons, promoting an increase in neuroprotective α-cleavage while reducing β-cleavage products, confirming the effectiveness of the CRISPR/Cas9 strategy at the cellular level (Adapted from Sun et al., 2018) |
Enhancing amyloid clearance is another critical strategy in gene therapy for AD. This can be achieved by upregulating genes involved in Aβ degradation or by modulating the immune system to enhance microglial activity, which is responsible for clearing amyloid plaques. Tau aggregation, which correlates more closely with cognitive decline than amyloid plaques, can be mitigated through gene therapies that stabilize microtubules or prevent tau from becoming hyperphosphorylated and forming tangles (Congdon and Sigurdsson, 2018).
3.2 Neuroprotective gene therapy
Neuroprotective gene therapy focuses on enhancing the expression of proteins that protect neurons from the toxic effects of Aβ and tau. One promising approach involves the delivery of genes encoding neuroprotective factors like brain-derived neurotrophic factor (BDNF) or nerve growth factor (NGF). NGF, in particular, has been shown to reduce amyloidogenesis and promote neuronal survival by interacting with the TrkA receptor, which is crucial for neuronal function (Triaca et al., 2016). Gene therapies that increase NGF expression in the brain have demonstrated the ability to reduce tau phosphorylation and prevent neurodegeneration, suggesting a potential therapeutic strategy for AD (Zhou et al., 2020).
3.3 Gene editing and CRISPR/Cas9
The advent of CRISPR/Cas9 technology has opened new avenues for gene therapy in AD by enabling precise editing of genes associated with the disease (Li and Li, 2024). CRISPR/Cas9 can be used to correct pathogenic mutations in genes such as APP, PSEN1, and PSEN2, thereby reducing the production of toxic Aβ peptides (Adji et al., 2022). Furthermore, CRISPR/Cas9 can be employed to modulate gene expression or to insert genes that confer neuroprotection, making it a versatile tool for addressing the complex genetic factors involved in AD (Lu et al., 2021).
However, the application of CRISPR/Cas9 in AD also raises significant ethical considerations and technical challenges. The potential for off-target effects, where unintended sections of DNA are edited, poses risks that need to be carefully managed. Moreover, the long-term implications of gene editing, particularly in the brain, are not fully understood, and there are concerns about the heritability of genetic modifications and their impact on future generations (Barman et al., 2020). These challenges underscore the need for thorough preclinical testing and stringent regulatory oversight to ensure the safety and efficacy of CRISPR/Cas9-based therapies for AD.
Gene therapy targeting the amyloid-beta and tau pathways, enhancing neuroprotective proteins, and utilizing CRISPR/Cas9 technology holds great promise for the treatment of Alzheimer's disease. As research progresses, these strategies may offer new hope in combating the devastating effects of AD, though careful consideration of the associated risks and ethical issues remains paramount.
4 Current Strategies in Gene Therapy for Alzheimer’s Disease
4.1 Viral vector-based therapies
Viral vectors have been at the forefront of gene therapy due to their high efficiency in delivering genetic material into target cells. Among these, adeno-associated virus (AAV) and lentivirus are particularly prominent in the context of Alzheimer’s disease (AD) research. AAV vectors are favored for their ability to infect both dividing and non-dividing cells, making them suitable for targeting neurons, which are largely non-dividing. AAV vectors are also known for their low immunogenicity and long-term expression of therapeutic genes (Ralph et al., 2006; Honig, 2018). Lentiviral vectors, on the other hand, have the capacity to integrate into the host genome, leading to stable and long-term expression of the therapeutic gene. This feature is particularly useful for chronic conditions like AD, where sustained gene expression is critical (Kumar and Woon-Khiong, 2011).
Despite the advantages, viral vectors face significant challenges in gene therapy for AD. One major challenge is the blood-brain barrier (BBB), which limits the efficient delivery of these vectors to the central nervous system (CNS). Strategies to overcome this include the development of vectors that can cross the BBB or the use of invasive methods like intracranial injections (Butt et al., 2022). Additionally, there are concerns regarding the potential for insertional mutagenesis with lentiviral vectors, where the integration of the viral genome into the host DNA could disrupt important genes and lead to oncogenesis. Advances in vector design, such as the use of integration-deficient lentiviral vectors, are being explored to mitigate these risks (Kumar and Woon-Khiong, 2011).
4.2 Non-viral gene delivery systems
Non-viral gene delivery systems, such as nanoparticles and liposomes, have gained traction due to their safety profile and flexibility. Nanoparticles can be engineered to bypass the BBB and deliver genes directly to the brain, reducing systemic toxicity and improving the specificity of gene delivery (Arora et al., 2021). Liposomes, which are lipid-based carriers, can encapsulate nucleic acids and protect them from degradation, allowing for efficient delivery to target cells. Both nanoparticles and liposomes can be modified with targeting ligands to enhance their delivery to specific cell types, including neurons affected by AD.
Recent advances in nanotechnology have led to the development of multifunctional nanoparticles that not only deliver genes but also facilitate their controlled release in the brain. These nanoparticles can be designed to respond to specific stimuli, such as pH or temperature changes, which are characteristic of diseased tissues, ensuring that the therapeutic genes are released only where they are needed (Ediriweera et al., 2021). Furthermore, nanotechnology allows for the co-delivery of multiple therapeutic agents, such as genes and drugs, which could provide a more comprehensive approach to treating AD by targeting multiple pathways simultaneously (Annu et al., 2022).
4.3 Gene therapy with stem cells
Gene therapy combined with stem cell technology offers a promising strategy for neuroregeneration in AD. Induced pluripotent stem cells (iPSCs) can be genetically modified to overexpress neuroprotective genes and then transplanted into the brain to replace lost or damaged neurons. These gene-modified stem cells not only integrate into the existing neural network but also provide a continuous source of therapeutic proteins that can mitigate the progression of AD (Haridhasapavalan et al., 2019).
The use of iPSCs, which are derived from a patient’s own cells, circumvents the ethical and immunological issues associated with embryonic stem cells. By using gene-editing tools like CRISPR/Cas9, iPSCs can be corrected for any genetic mutations before being differentiated into neurons and transplanted back into the patient’s brain. This approach not only aims to replace lost neurons but also to correct the underlying genetic causes of AD, offering a potentially curative treatment (Komatsu et al., 2019). However, the clinical translation of these therapies requires further research to ensure the safety and efficacy of the genetically modified cells, particularly in terms of their long-term integration and function in the brain.
5 Current Research and Clinical Trials
5.1 Preclinical studies
Preclinical studies in animal models of Alzheimer's disease (AD) have provided valuable insights into the potential efficacy of gene therapy. These models, primarily transgenic mice that express human genes associated with familial AD, have been instrumental in evaluating the effects of gene therapy interventions. For instance, the use of adeno-associated virus (AAV) vectors to deliver neuroprotective genes such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) has shown promise in reducing amyloid-beta plaque formation and enhancing neuronal survival (Tuszynski et al., 2015). Similarly, gene therapy approaches targeting the tau protein have demonstrated efficacy in reducing tau pathology and improving cognitive function in these models (Tedeschi et al., 2021). These successes highlight the potential of gene therapy as a treatment for AD.
Despite the promising results in animal models, translating these findings to human clinical trials has been fraught with challenges. One major issue is the difference in disease pathology between animals and humans, which can result in therapies that are effective in animal models failing to show similar efficacy in humans. For example, the complexity of the human brain and the multifactorial nature of AD mean that therapies targeting a single pathway may not be sufficient to halt disease progression (Banik et al., 2015). Additionally, the blood-brain barrier (BBB) poses a significant hurdle in delivering gene therapies effectively to the human brain, a challenge that is less pronounced in animal models (LaFerla and Green, 2012).
5.2 Completed and ongoing clinical trials
Several clinical trials have been initiated to test the safety and efficacy of gene therapies in AD patients. Notably, early trials focused on the delivery of NGF to the brain using AAV vectors. Although these trials demonstrated the safety of the approach, the results were inconclusive regarding cognitive benefits, partly due to challenges in achieving adequate vector delivery and gene expression in target areas (Castle et al., 2020). More recent trials are exploring the use of advanced delivery methods, including convection-enhanced delivery and MRI-guided stereotactic surgery, to improve the accuracy and efficacy of gene delivery (Tuszynski et al., 2015).
The outcomes of these clinical trials have highlighted several challenges in the application of gene therapy for AD. One of the primary challenges has been the limited spread of the therapeutic gene within the brain, resulting in suboptimal engagement of target neurons. Furthermore, the heterogeneity of AD, both in terms of genetic mutations and disease progression, complicates the design of clinical trials and the interpretation of results (Yiannopoulou et al., 2019). Despite these challenges, ongoing trials continue to refine gene delivery techniques and explore combination therapies to enhance therapeutic outcomes.
5.3 Emerging research trends
To address the challenges observed in earlier trials, researchers are investigating new viral vectors and delivery methods that can achieve more widespread and targeted gene expression in the brain. For example, the development of modified AAV vectors with enhanced tropism for neurons and the use of non-viral delivery systems such as nanoparticles are promising approaches under investigation (Chen et al., 2020). Additionally, advances in real-time imaging techniques, such as MRI-guided delivery, are being incorporated into clinical trial designs to improve the precision of gene therapy administration.
Emerging research is also focusing on identifying novel therapeutic targets for gene therapy in AD. Beyond amyloid-beta and tau, researchers are exploring the potential of targeting neuroinflammation, synaptic dysfunction, and oxidative stress, all of which play critical roles in AD pathogenesis (Owens et al., 2021). These efforts are aimed at developing multifaceted gene therapy approaches that can address the various aspects of AD pathology, offering a more comprehensive treatment strategy.
6 Challenges and Limitations of Gene Therapy in AD
6.1 Technical and biological barriers
One of the most significant technical challenges in applying gene therapy for Alzheimer's disease (AD) is the blood-brain barrier (BBB). The BBB is a selective barrier that protects the brain from potentially harmful substances in the bloodstream but also significantly limits the ability of therapeutic agents, including viral and non-viral vectors, to reach the brain in adequate concentrations. Strategies to overcome this include using modified viral vectors like adeno-associated viruses (AAVs) that can cross the BBB more effectively, and employing non-viral delivery systems such as nanoparticles that are designed to bypass or transiently disrupt the BBB (Weber-Adrian, 2019). However, ensuring that these therapies reach specific regions of the brain affected by AD while minimizing off-target effects remains a formidable challenge (Arora et al., 2021).
Another major concern is the long-term safety and efficacy of gene therapy. Ensuring sustained expression of the therapeutic gene over time without triggering an immune response or causing toxicity is critical. The risk of insertional mutagenesis, where the integration of the therapeutic gene disrupts essential host genes, remains a significant safety concern, particularly with viral vectors that integrate into the host genome, like lentiviruses (Chen et al., 2020). Non-integrating vectors or episomal vectors may offer a safer alternative, but they often suffer from reduced expression over time, requiring repeated administration, which may not be feasible or desirable in a chronic condition like AD (Tedeschi et al., 2021).
6.2 Ethical considerations
The application of gene editing technologies, particularly CRISPR/Cas9, in treating AD raises significant ethical concerns. The possibility of off-target effects and the long-term implications of gene edits, especially if they are heritable, necessitate rigorous ethical scrutiny. Moreover, the need for long-term monitoring of patients who receive gene therapy to track the persistence and effects of the therapy, including any unintended consequences, adds another layer of complexity. The potential for unforeseen side effects that may only become apparent years after treatment highlights the ethical obligation to ensure thorough and long-term follow-up.
Public perception of gene therapy is influenced by concerns over safety, ethics, and the potential for misuse, such as in germline editing. This, combined with the complex regulatory landscape that governs the approval of gene therapies, presents significant hurdles. Regulatory bodies require extensive evidence of safety and efficacy, which can delay the availability of these therapies to patients who need them. Moreover, the ethical issues surrounding consent, especially in patients with cognitive impairment, pose challenges in the context of clinical trials and treatment implementation (Davis, 2017).
6.3 Clinical efficacy and cost
Gene therapy is one of the most expensive forms of treatment due to the complex processes involved in vector production, delivery, and patient monitoring. This high cost poses a significant barrier to widespread adoption, especially when the clinical outcomes are still under investigation. Balancing the enormous costs associated with gene therapy against the potential, but as yet unproven, benefits is a significant challenge for healthcare systems. The cost-effectiveness of gene therapy in AD will need to be thoroughly evaluated, considering the long-term benefits and the reduction in care costs that successful treatment could bring (Shellhaas et al., 2021).
Another significant challenge is demonstrating the long-term efficacy of gene therapy across diverse patient populations. The heterogeneity of AD, with its varying genetic, environmental, and lifestyle factors, makes it difficult to predict how different individuals will respond to the same gene therapy. Clinical trials need to account for this variability, but doing so increases the complexity and cost of the trials. Moreover, long-term studies are required to assess the sustained benefits of gene therapy, which can be logistically and financially challenging (Yiannopoulou et al., 2019).
While gene therapy holds great promise for treating Alzheimer's disease, it faces significant technical, ethical, and economic challenges that must be addressed. Overcoming these barriers will be crucial for realizing the full potential of gene therapy in providing long-term, effective treatments for AD.
7 Future Directions and Emerging Trends
7.1 Advancements in gene editing technologies
The CRISPR/Cas9 gene-editing technology has emerged as a powerful tool for precisely targeting and modifying specific genetic sequences associated with Alzheimer's disease (AD). Its potential applications in AD therapy are vast, ranging from correcting pathogenic mutations in genes such as APP, PSEN1, and APOE to modulating gene expression to prevent the formation of amyloid-beta plaques and neurofibrillary tangles. Recent studies have demonstrated the ability of CRISPR/Cas9 to reduce amyloid-beta production and improve cognitive function in AD mouse models, paving the way for potential human applications (Rohn et al., 2018). However, the clinical translation of CRISPR/Cas9 remains challenging due to concerns about off-target effects and the need for safe and effective delivery systems to the brain (Bhardwaj et al., 2021).
Advances in nanotechnology are contributing to the development of next-generation gene delivery systems that can overcome the limitations of current CRISPR/Cas9 applications in AD. Nanoparticles and other non-viral vectors are being explored as alternatives to traditional viral vectors, offering the potential for safer and more precise delivery of gene-editing tools. These systems can be engineered to cross the blood-brain barrier and deliver CRISPR/Cas9 components directly to the affected regions of the brain, minimizing systemic exposure and reducing the risk of off-target effects (Hanafy et al., 2020).
7.2 Personalized gene therapy
Personalized medicine is an emerging trend in gene therapy, particularly in the treatment of complex diseases like AD. By tailoring gene therapy approaches to an individual’s genetic profile, it is possible to achieve more effective and targeted treatments (Zhang, 2024). For instance, individuals with specific mutations in the APP or PSEN1 genes might benefit from therapies designed to correct these mutations using CRISPR/Cas9, while others with different genetic risk factors may require alternative approaches. This level of personalization holds the promise of optimizing therapeutic outcomes and minimizing adverse effects (Paquet et al., 2016; Adji et al., 2022).
Another promising direction is the combination of gene therapy with other therapeutic modalities, such as immunotherapy, pharmacotherapy, or lifestyle interventions. Combining CRISPR/Cas9-based gene editing with drugs that target amyloid-beta or tau pathways, for example, could provide a more comprehensive approach to treating AD. Additionally, gene therapy might be used to enhance the efficacy of traditional treatments, such as by increasing the expression of drug targets or by protecting neurons from the toxic effects of amyloid-beta and tau (Barman et al., 2020; Lu et al., 2021).
7.3 Exploration of new therapeutic targets
While amyloid-beta and tau have been the primary targets of AD research, emerging evidence suggests that other pathways, such as neuroinflammation and neuroprotection, may also play critical roles in the disease. Gene therapies that target these pathways could provide new avenues for treatment. For example, modulating the expression of genes involved in neuroinflammatory responses or enhancing the production of neuroprotective factors like BDNF could help slow the progression of AD and protect against neuronal loss (Hanafy et al., 2020; Lu et al., 2021).
The interplay between genetics, environment, and lifestyle is increasingly recognized as a key factor in the development and progression of AD. Future gene therapy research may focus on understanding how environmental factors such as diet, exercise, and exposure to toxins interact with genetic predispositions to influence disease risk. This knowledge could lead to the development of gene therapies that are not only tailored to an individual’s genetic profile but also take into account their environmental exposures and lifestyle choices, offering a more holistic approach to AD prevention and treatment (Adji et al., 2022).
The future of gene therapy for Alzheimer's disease lies in the continued advancement of gene-editing technologies, the development of personalized approaches, and the exploration of new therapeutic targets. These emerging trends hold the promise of transforming the treatment landscape for AD, offering hope for more effective and enduring interventions.
8 Concluding Remarks
Gene therapy represents a transformative approach in the treatment of Alzheimer's disease (AD), offering the potential to address the underlying genetic and molecular causes of this complex neurodegenerative disorder. By directly targeting pathogenic genes, such as APP, PSEN1, and APOE, gene therapy can potentially halt or even reverse the progression of AD, rather than merely alleviating symptoms. Techniques like CRISPR/Cas9 have shown promise in preclinical models, where they have successfully reduced amyloid-beta production and improved cognitive functions. Additionally, the development of advanced delivery systems, including viral vectors and nanotechnology-based approaches, has enhanced the precision and efficacy of gene therapies in reaching target brain regions while minimizing off-target effects.
Despite the promising developments, several challenges remain that must be addressed through future research. First, improving the safety and efficiency of gene delivery systems is crucial, particularly in overcoming the blood-brain barrier and achieving sustained gene expression without adverse effects. Additionally, the potential for off-target effects with gene-editing technologies like CRISPR/Cas9 must be minimized through advancements in vector design and targeting accuracy. Research should also focus on identifying new therapeutic targets beyond amyloid-beta and tau, including pathways related to neuroinflammation, oxidative stress, and synaptic dysfunction, which could offer broader treatment options for AD. Moreover, personalized gene therapy approaches that consider individual genetic profiles, environmental factors, and lifestyle choices could enhance the efficacy and safety of these treatments, providing tailored interventions for patients.
Translating the advances in gene therapy for AD from the laboratory to the clinic involves significant hurdles. Regulatory challenges, ethical considerations, and the high costs associated with gene therapy development are substantial barriers to widespread clinical adoption. Long-term studies are required to evaluate the efficacy, safety, and durability of gene therapies in diverse patient populations. Additionally, the integration of gene therapy with other treatment modalities, such as pharmacotherapy and lifestyle interventions, will be critical in achieving comprehensive and effective care for AD patients. Collaboration between researchers, clinicians, regulatory bodies, and industry partners will be essential to overcome these obstacles and ensure that gene therapy becomes a viable and accessible option for those affected by AD.
In conclusion, while significant challenges remain, the prospects of gene therapy in Alzheimer's disease are promising, offering the potential to fundamentally change the treatment landscape for this devastating condition. Continued research, innovation, and collaboration will be key to realizing the full potential of gene therapy in AD, ultimately leading to more effective and personalized treatments for patients.
Acknowledgments
The authors express gratitude to the two anonymous peer reviewers for their feedback.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Adji A., Widjaja J., Wardani V., Muhammad A., Handajani F., Putra H., and Rahman F., 2022, A review of CRISPR Cas9 for Alzheimer's disease: treatment strategies and could target APOE e4, APP, and PSEN-1 Gene using CRISPR cas9 prevent the patient from alzheimer's disease? Open Access Macedonian Journal of Medical Sciences, 10(F): 745-57.
https://doi.org/10.3889/oamjms.2022.9053
Almkvist O., and Graff C., 2021, The APOE ε4 allele affects cognitive functions differently in carriers of APP mutations compared to carriers of PSEN1 mutations in Autosomal-Dominant Alzheimer's disease, Genes, 12(12): 1954.
https://doi.org/10.3390/genes12121954
PMid:34946903 PMCid:PMC8701239
Annu A., Rehman S., Nabi B., Sartaj A., Baboota S., Md S., Sahoo P., and Ali J., 2022, Nanoparticle mediated gene therapy: a trailblazer armament to fight CNS disorders, Current Medicinal Chemistry, 30(3): 304-315.
https://doi.org/10.2174/0929867329666220105122318
PMid:34986767
Arora S., Sharma D., Layek B., and Singh J., 2021, A review of brain-targeted nonviral gene-based therapies for the treatment of Alzheimer's disease, Molecular Pharmaceutics, 18(12): 4237-4255.
https://doi.org/10.1021/acs.molpharmaceut.1c00611
Banik A., Brown R., Bamburg J., Lahiri D., Khurana D., Friedland R., Chen W., Ding Y., Mudher A., Padjen A., Mukaetova-Ladinska E., Ihara M., Srivastava S., Srivastava M., Masters C., Kalaria R., and Anand A., 2015, Translation of pre-clinical studies into successful clinical trials for Alzheimer's disease: what are the roadblocks and how can they be overcome? Journal of Alzheimer's Disease, 47(4): 815-843.
https://doi.org/10.3233/JAD-150136
PMid:26401762
Barman N., Khan N., Islam M., Nain Z., Roy R., Haque A., and Barman S., 2020, CRISPR-Cas9: a promising genome editing therapeutic tool for Alzheimer's disease-a narrative review, Neurology and Therapy, 9: 419-434.
https://doi.org/10.1007/s40120-020-00218-z
PMid:33089409 PMCid:PMC7606404
Bhardwaj S., Kesari K., Rachamalla M., Mani S., Ashraf G., Jha S., Kumar P., Ambasta R., Dureja H., Devkota H., Gupta G., Chellappan D., Singh S., Dua K., Ruokolainen J., Kamal M., Ojha S., and Jha N., 2021, CRISPR/Cas9 gene editing: new hope for Alzheimer's disease therapeutics, Journal of Advanced Research, 40: 207-221.
https://doi.org/10.1016/j.jare.2021.07.001
PMid:36100328 PMCid:PMC9481950
Breijyeh Z., and Karaman R., 2020, Comprehensive review on Alzheimer's disease: causes and treatment, Molecules, 25(24): 5789.
https://doi.org/10.3390/molecules25245789
PMid:33302541 PMCid:PMC7764106
Butt M., Zaman M., Ahmad A., Khan R., Mallhi T., Hasan M., Khan Y., Hafeez S., Massoud E., Rahman M., and Cavalu S., 2022, Appraisal for the potential of viral and nonviral vectors in gene therapy: a review, Genes, 13(8): 1370.
https://doi.org/10.3390/genes13081370
PMid:36011281 PMCid:PMC9407213
Castle M., Baltanás F., Kovács I., Nagahara A., Barba D., and Tuszynski M., 2020, Postmortem analysis in a clinical trial of AAV2-NGF gene therapy for alzheimer's disease identifies a need for improved vector delivery, Human Gene Therapy, 31(7-8): 415-422.
https://doi.org/10.1089/hum.2019.367
PMid:32126838 PMCid:PMC7194314
Chen W., Hu Y., and Ju D., 2020, Gene therapy for neurodegenerative disorders: advances, insights and prospects, Acta Pharmaceutica Sinica B, 10: 1347-1359.
https://doi.org/10.1016/j.apsb.2020.01.015
PMid:32963936 PMCid:PMC7488363
Congdon E., and Sigurdsson E., 2018, Tau-targeting therapies for Alzheimer disease, Nature Reviews Neurology, 14: 399-415.
https://doi.org/10.1038/s41582-018-0013-z
PMid:29895964 PMCid:PMC6463489
Costa-Laparra I., Juárez-Escoto E., Vicario C., Moratalla R., and García-Sanz P., 2023, APOE ε4 allele, along with G206D-PSEN1 mutation, alters mitochondrial networks and their degradation in Alzheimer's disease, Frontiers in Aging Neuroscience, 15: 1087072.
https://doi.org/10.3389/fnagi.2023.1087072
PMid:37455931 PMCid:PMC10340123
Davis D., 2017, Ethical issues in Alzheimer's disease research involving human subjects, Journal of Medical Ethics, 43: 852-856.
https://doi.org/10.1136/medethics-2016-103392
PMid:28798225
Ediriweera G., Chen L., Yerbury J., Thurecht K., and Vine K., 2021, Non-viral vector-mediated gene therapy for ALS: challenges and future perspectives, Molecular Pharmaceutics, 18(6): 2142-2160.
https://doi.org/10.1021/acs.molpharmaceut.1c00297
PMid:34010004
Gao Y., Ren R., Zhong Z., Dammer E., Zhao Q., Shan S., Zhou Z., Li X., Zhang Y., Cui H., Hu Y., Chen S., Chen J., Guo Q., and Wang G., 2019, Mutation profile of APP, PSEN1, and PSEN2 in Chinese familial Alzheimer's disease, Neurobiology of Aging, 77: 154-157.
https://doi.org/10.1016/j.neurobiolaging.2019.01.018
PMid:30822634
Ghaffari M., Sanadgol N., and Abdollahi M., 2020, A systematic review of current progresses in the nucleic acid-based therapies for neurodegeneration with Implications for Alzheimer's disease, Mini Reviews in Medicinal Chemistry, 20(15): 1499-1517.
https://doi.org/10.2174/1389557520666200513122357
PMid:32400332
Giau V., Bagyinszky E., Youn Y., An S., and Kim S., 2019, APP, PSEN1, and PSEN2 mutations in Asian patients with early-onset Alzheimer disease, International Journal of Molecular Sciences, 20(19): 4757.
https://doi.org/10.3390/ijms20194757
PMid:31557888 PMCid:PMC6801447
Guven G., Samancı B., Gulec C., Hanagasi H., Gurvit H., Gokalp E., Tepgec F., Guler S., Uyguner O., and Bilgiç B., 2021, A novel PSEN2 p.Ser175Phe variant in a family with Alzheimer's disease, Neurological Sciences, 42: 2497-2504.
https://doi.org/10.1007/s10072-021-05243-w
PMid:33855622
Hanafy A., Schoch S., and Lamprecht A., 2020, CRISPR/Cas9 delivery potentials in Alzheimer's disease management: a mini review, Pharmaceutics, 12(9): 108.
https://doi.org/10.3390/pharmaceutics12090801
PMid:32854251 PMCid:PMC7559557
Haridhasapavalan K., Borgohain M., Dey C., Saha B., Narayan G., Kumar S., and Thummer R., 2019, An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells, Gene, 686: 146-159.
https://doi.org/10.1016/j.gene.2018.11.069
PMid:30472380
Honig L., 2018, Gene therapy in alzheimer disease-it may be feasible, but will it be beneficial? JAMA Neurology, 75(7): 791-793.
https://doi.org/10.1001/jamaneurol.2017.4029
PMid:29582049
Kent S., Spires-Jones T., and Durrant C., 2020, The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics, Acta Neuropathologica, 140: 417-447.
https://doi.org/10.1007/s00401-020-02196-w
PMid:32728795 PMCid:PMC7498448
Komatsu Y., Takeuchi D., Tokunaga T., Sakurai H., Makino A., Honda T., Ikeda Y., and Tomonaga K., 2019, RNA virus-based episomal vector with a fail-safe switch facilitating efficient genetic modification and differentiation of iPSCs, Molecular Therapy Methods and Clinical Development, 14: 47-55.
https://doi.org/10.1016/j.omtm.2019.05.010
PMid:31309127 PMCid:PMC6606997
Kumar P., and Woon-Khiong C., 2011, Optimization of lentiviral vectors generation for biomedical and clinical research purposes: contemporary trends in technology development and applications, Current Gene Therapy, 11(2): 144-153.
https://doi.org/10.2174/156652311794940782
PMid:21291355
Lacour M., Quenez O., Rovelet-Lecrux A., Salomon B., Rousseau S., Richard A., Quillard‐Muraine M., Pasquier F., Rollin-Sillaire A., Martinaud O., Zaréa A., Sayette V., Boutoleau-Bretonnière C., Etcharry-Bouyx F., Chauviré V., Sarazin M., Ber I., Epelbaum S., Jonveaux T., Rouaud O., Ceccaldi M., Godefroy O., Formaglio M., Croisile B., Auriacombe S., Magnin E., Sauvée M., Marelli C., Gabelle A., Pariente J., Paquet C., Boland A., Deleuze J., Campion D., Hannequin D., Nicolas G., and Wallon D., 2019, Causative mutations and genetic risk factors in sporadic early onset alzheimer's disease before 51 years, Journal of Alzheimer's Disease, 71(1): 227-243.
https://doi.org/10.3233/JAD-190193
PMid:31381512
LaFerla F., and Green K., 2012, Animal models of Alzheimer disease, Cold Spring Harbor perspectives in medicine, 2: 11.
https://doi.org/10.1101/cshperspect.a006320
PMid:23002015 PMCid:PMC3543097
Li J.H., and Li J., 2024, Gene editing for organ transplants: evaluating the impact of CRISPR/Cas9 on immunogenicity and organ longevity in pigs, International Journal of Clinical Case Reports, 14(2): 94-106.
Loera-Valencia R., Piras A., Ismail M., Manchanda S., Eyjólfsdóttir H., Saido T., Johansson J., Eriksdotter M., Winblad B., and Nilsson P., 2018, Targeting Alzheimer's disease with gene and cell therapies, Journal of Internal Medicine, 284: 2-36.
https://doi.org/10.1111/joim.12759
PMid:29582495
Lu L., Yu X., Cai Y., Sun M., and Yang H., 2021, Application of CRISPR/Cas9 in Alzheimer's disease, Frontiers in Neuroscience, 15: 803894.
https://doi.org/10.3389/fnins.2021.803894
PMid:34992519 PMCid:PMC8724030
Owens L., Benedetto A., Dawson N., Gaffney C., and Parkin E., 2021, Gene therapy-mediated enhancement of protective protein expression for the treatment of Alzheimer's disease, Brain Research, 1753: 147264.
https://doi.org/10.1016/j.brainres.2020.147264
PMid:33422539
Paquet D., Kwart D., Chen A., Sproul A., Jacob S., Teo S., Olsen K., Gregg A., Noggle S., and Tessier-Lavigne M., 2016, Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9, Nature, 533, 125-129.
https://doi.org/10.1038/nature17664
PMid:27120160
Ralph G., Binley K., Wong L., Azzouz M., and Mazarakis N., 2006, Gene therapy for neurodegenerative and ocular diseases using lentiviral vectors, Clinical Science, 110(1): 37-46.
https://doi.org/10.1042/CS20050158
PMid:16336203
Roda A., Serra-Mir G., Montoliu-Gaya L., Tiessler L., and Villegas S., 2022, Amyloid-beta peptide and tau protein crosstalk in Alzheimer's disease, Neural Regeneration Research, 17: 1666-1674.
https://doi.org/10.4103/1673-5374.332127
PMid:35017413 PMCid:PMC8820696
Rohn T., Kim N., Isho N., and Mack J., 2018, The potential of CRISPR/Cas9 gene editing as a treatment strategy for alzheimer's disease, Journal of Alzheimer's Disease and Parkinsonism, 8: 1000439.
https://doi.org/10.4172/2161-0460.1000439
PMid:30090689 PMCid:PMC6078432
Rudenko Y., and Sholomon S., 2023, Prospects of using gene therapy and nanotechnologies for the treatment of neurodegenerative diseases, Ukrainian Neurological Journal, 2023: 45.
https://doi.org/10.30978/UNJ2023-1-4-45
Shellhaas R., deVeber G., Bonkowsky J., Augustine E., Bassuk A., Calame D., Carrasco M., Dlamini N., Felling R., Glass H., Grinspan Z., Guerriero R., Hewitt A., Jeste S., Knowles J., Lyons-Warren A., Maricich S., Musolino P., Raju G., Rho J., Rotenberg A., Sherr E., Soul J., and Ziobro J., 2021, Gene-targeted therapies in pediatric neurology: challenges and opportunities in diagnosis and delivery, Pediatric Neurology, 125: 53-57.
https://doi.org/10.1016/j.pediatrneurol.2021.09.011
Shim K., Kang S., An S., and Kang M., 2022, Identification of the third case of PSEN1 Tyr389His variant in early-onset alzheimer's disease in Korea, International Journal of Molecular Sciences, 23(24): 16192.
https://doi.org/10.3390/ijms232416192
PMid:36555832 PMCid:PMC9781446
Sudhakar V., and Richardson R., 2018, Gene therapy for neurodegenerative diseases, Neurotherapeutics, 16: 166-175.
https://doi.org/10.1007/s13311-018-00694-0
PMid:30542906 PMCid:PMC6361055
Sun J., Carlson-Stevermer J., Das U., Shen M., Delenclos M., Snead A., Wang L., Loi J., Petersen A., Stockton M., Bhattacharyya A., Jones M., Sproul A., McLean P., Zhao X., Saha K., and Roy S., 2018, A CRISPR/Cas9 based strategy to manipulate the Alzheimer's amyloid pathway, bioRxiv, 28: 310193.
https://doi.org/10.1101/310193
Tedeschi D., Cunha A., Cominetti M., and Pedroso R., 2021, Efficacy of gene therapy to restore cognition in Alzheimer's disease: a systematic review, Current Gene Therapy, 21(3): 246-257.
https://doi.org/10.2174/1566523221666210120091146
Triaca V., Sposato V., Bolasco G., Ciotti M., Pelicci P., Bruni A., Cupidi C., Maletta R., Feligioni M., Nisticò R., Canu N., and Calissano P., 2016, NGF controls APP cleavage by downregulating APP phosphorylation at Thr668: relevance for Alzheimer's disease, Aging Cell, 15: 661-672.
https://doi.org/10.1111/acel.12473
PMid:27076121 PMCid:PMC4933663
Tuszynski M., Yang J., Barba D., U H., Bakay R., Pay M., Masliah E., Conner J., Kobalka P., Roy S., and Nagahara A., 2015, Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease, JAMA Neurology, 72(10): 1139-1147.
https://doi.org/10.1001/jamaneurol.2015.1807
PMid:26302439 PMCid:PMC4944824
Unnisa A., Greig N., and Kamal M., 2023, Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer's disease, Neural Regeneration Research, 18: 2127-2133.
https://doi.org/10.4103/1673-5374.369096
PMid:37056119 PMCid:PMC10328264
Weber-Adrian D., 2019, Gene therapy: a strategy for the treatment of Alzheimer's disease, Health Science Inquiry, 6(1):23.
https://doi.org/10.29173/hsi190
Yiannopoulou K., Anastasiou A., Zachariou V., and Pelidou S., 2019, Reasons for failed trials of disease-modifying treatments for alzheimer disease and their contribution in recent research, Biomedicines, ;7(4): 97.
https://doi.org/10.3390/biomedicines7040097
Zhang J., 2024, From genomic data to personalized medical decisions: challenges and opportunities, International Journal of Clinical Case Reports, 14(2): 107-116.
Zhou H., Gong Y., Liu Y., Huang A., Zhu X., Liu J., Yuan G., Zhang L., Wei J., and Liu J., 2020, Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer's disease, Biomaterials, 237: 119822.
https://doi.org/10.1016/j.biomaterials.2020.119822
(1).png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yuchuan Yang
. Xiaoying Xu
Related articles
. Alzheimer's disease
. Gene therapy
. CRISPR/Cas9
. Amyloid-β
. Personalized medicine
Tools
. Post a comment