Author
Author  Correspondence author
Correspondence author
Journal of Vaccine Research, 2024, Vol. 14, No. 5
Received: 15 Jul., 2024 Accepted: 31 Aug., 2024 Published: 07 Sep., 2024
The development of multi-pathogen vaccines offers a promising approach to improving global immunization efforts by providing protection against multiple infectious pathogens with a single formulation. This study comprehensively evaluates the safety and efficacy of multi-pathogen vaccines, examining their historical development, biological and immunological foundations, and key case studies. While multi-pathogen vaccines have significant advantages in simplifying immunization schedules, reducing healthcare costs, and increasing vaccine coverage, they also face unique challenges such as immune interference, production complexity, and regulatory hurdles. This study discusses strategies to enhance the safety and efficacy of these vaccines, including the use of advanced adjuvants, optimized dosing regimens, novel delivery systems, and synergistic agents. The research provides valuable insights into the opportunities and challenges of multi-pathogen vaccines and emphasizes their potential role in addressing the global burden of infectious diseases. Keywords Multi-pathogen vaccines; Vaccine safety; Vaccine efficacy; Immune interference; mRNA technology
1 Introduction
In recent years, the development of multi-pathogen vaccines has gained considerable attention due to their potential to provide protection against multiple infectious agents in a single formulation. These vaccines, designed to target more than one pathogen simultaneously, offer significant advantages in global health efforts, particularly in regions with limited healthcare access and high disease burdens (Sharma et al., 2021). Studies on vaccines such as the multi-dose PfSPZ Vaccine and other combined vaccines have shown promise in protecting against multiple diseases effectively (Voysey et al., 2020; Jongo et al., 2022). The convenience of a single-dose vaccine capable of immunizing against various diseases can lead to improved vaccination rates, reduced costs, and simplified immunization schedules.
However, the safety and efficacy of multi-pathogen vaccines are critical concerns that must be thoroughly assessed to ensure their successful implementation. Vaccine safety refers to the ability to induce immunity without causing significant adverse effects, while efficacy ensures the vaccine’s capability to offer adequate protection against targeted diseases (Zhou et al., 2021; Xu et al., 2024). These factors are paramount for public acceptance and widespread use, making it essential to evaluate multi-pathogen vaccines rigorously (Folegatti et al., 2020). Trials of vaccines like the IC43 Pseudomonas aeruginosa vaccine and others demonstrate the importance of ensuring vaccines are both immunogenic and safe, even in high-risk populations (Adlbrecht et al., 2020; Logunov et al., 2021).
This study will conduct a comprehensive analysis of the safety and efficacy of existing multi-pathogen vaccines. By reviewing current research and clinical trial data that demonstrate their effectiveness and safety, this study highlights the potential benefits and challenges of multi-pathogen vaccines. In the context of combating emerging infectious diseases and achieving broad-spectrum protection, the findings provide guidance for future vaccine development strategies.
2 Historical Overview of Multi-Pathogen Vaccine Development
2.1 Evolution of vaccine development
The development of vaccines has undergone significant evolution since the first successful vaccine, which was the smallpox vaccine introduced by Edward Jenner in the late 18th century (Figure 1). Traditional vaccines were designed to target a single pathogen by utilizing weakened or killed forms of the disease-causing organism (Pastural et al., 2019). As understanding of immunology expanded, vaccine science progressed from basic inoculations to more sophisticated approaches, such as subunit vaccines, vector-based vaccines, and mRNA vaccines, which utilize genetic material to stimulate immune responses (Fierro et al., 2023). These advancements paved the way for the development of multi-pathogen vaccines, which aim to protect against multiple infectious agents in a single formulation (Folegatti et al., 2020).
|
Figure 1 Timeline of Vaccine Development History (Adapted from Saleh et al., 2021) Image caption: The timeline begins with Edward Jenner's smallpox vaccine in 1796 and extends to the COVID-19 vaccines. It highlights various landmark vaccines and their respective development dates, including polio, influenza, and HPV vaccines. Each vaccine invention represents significant scientific breakthroughs, such as the use of cell culture technology and genetic recombination, which have greatly advanced the progress of vaccine development (Adapted from Saleh et al., 2021) |
The concept of multi-pathogen vaccines emerged in response to the need for broader protection, particularly in areas where multiple infectious diseases are endemic. The introduction of combination vaccines, such as the DTP (diphtheria, tetanus, and pertussis) vaccine, marked a significant milestone in simplifying immunization schedules and improving public health outcomes. The shift towards targeting multiple pathogens in a single shot was driven by the desire to increase vaccine coverage, reduce healthcare costs, and improve compliance, especially in low-resource settings (Voysey et al., 2020).
2.2 Key milestones in the creation and deployment of multi-pathogen vaccines
Several key milestones have defined the trajectory of multi-pathogen vaccine development. The combination vaccine DTP, introduced in the mid-20th century, was among the first vaccines to successfully combine protection against multiple diseases (Jr Frenck et al., 2019). The development of the MMR (measles, mumps, rubella) vaccine further solidified the viability of multi-pathogen vaccines and their role in pediatric immunization programs. These early vaccines not only proved effective but also demonstrated that multi-pathogen approaches could be safely implemented in large populations (Sekuloski et al., 2018).
More recently, the advent of DNA and RNA-based vaccines has opened new possibilities for multi-pathogen vaccines. For example, the rapid development of mRNA vaccines for COVID-19 has shown that it is feasible to create vaccines that target several strains or even different viruses with the same platform (Zhu et al., 2020). These advancements indicate that the next generation of multi-pathogen vaccines may be able to address a broader range of infectious diseases, potentially combining vaccines for respiratory infections, influenza, and even coronaviruses into one formulation.
2.3 Challenges encountered in the early development of multi-pathogen vaccines
The early development of multi-pathogen vaccines was fraught with challenges. One of the primary hurdles was ensuring that combining antigens from multiple pathogens would not reduce the immune response to any single pathogen, a phenomenon known as immune interference. This was particularly evident in early trials of combination vaccines, where concerns about reduced efficacy led to significant delays in development (Malfertheiner et al., 2018).
Another challenge was the increased complexity of manufacturing multi-pathogen vaccines, which required the integration of different types of pathogens, proteins, or genetic material into a single vaccine. This complexity often resulted in stability issues and difficulty in maintaining consistent immune responses across all targeted pathogens. Additionally, safety concerns arose, as combining vaccines increased the potential for adverse reactions or side effects, which necessitated extensive testing and regulatory oversight (Baden et al., 2020).
Despite these challenges, the progress in multi-pathogen vaccines has been significant. Modern advances in immunology, molecular biology, and vaccine technologies have addressed many of these early obstacles, paving the way for safer and more effective vaccines. However, the continued development of these vaccines must remain vigilant to both immunological and logistical complexities to ensure their global applicability and success.
3 Biology and Immunological Basis of Multi-Pathogen Vaccines
3.1 Overview of the immune response
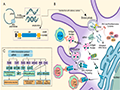
The immune response is the body's defense mechanism against pathogens, initiated through innate and adaptive immune pathways (Figure 2) (Xu et al., 2020; Xu and Li, 2024). Upon encountering an infectious agent, innate immune cells such as macrophages and dendritic cells identify pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs). This leads to the activation of innate immune responses, including the release of cytokines and chemokines, which create an inflammatory environment that helps recruit immune cells to the site of infection. In parallel, antigen-presenting cells (APCs) process and present antigens to T cells, activating the adaptive immune system (Lacaille-Dubois, 2019).
|
Figure 2 In Vitro Transcription Process of mRNA Vaccines and Their Activation Mechanism in Innate Immunity (Adapted from Xu et al., 2020) Image caption: After entering the cytoplasm through endocytosis, mRNA vaccines are recognized by various pattern recognition receptors (PRRs). Toll-like receptors (TLR7 and TLR8) recognize single-stranded RNA (ssRNA), while TLR3, cytoplasmic protein kinase R (PKR), and retinoic acid-inducible gene I (RIG-I) recognize double-stranded RNA (dsRNA). These recognition events activate antigen-presenting cells (APCs), leading to the secretion of type I interferon (IFN) and pro-inflammatory cytokines, which subsequently initiate an immune response (Adapted from Xu et al., 2020) |
The adaptive immune response involves two key branches: humoral and cell-mediated immunity. Humoral immunity, mediated by B cells, results in the production of pathogen-specific antibodies. These antibodies neutralize pathogens by binding to their surface antigens, preventing their entry into host cells (Lynn et al., 2021). Cell-mediated immunity, on the other hand, is driven by T cells, which can either help coordinate the immune response (helper T cells) or directly kill infected cells (cytotoxic T cells). This combination of innate and adaptive responses creates a robust defense against pathogens and forms the basis for vaccine-induced protection (Zhu et al., 2020).
3.2 Mechanism of action of multi-pathogen vaccines
Multi-pathogen vaccines aim to elicit protective immune responses against several different pathogens simultaneously. These vaccines typically include multiple antigens from different pathogens or a combination of genetic material encoding several distinct antigens. The mechanism of action of multi-pathogen vaccines involves the stimulation of both humoral and cell-mediated immune responses to each pathogen within the vaccine (Chen et al., 2023).
When a multi-pathogen vaccine is administered, the APCs process the diverse antigens and present them to T cells, leading to the activation of both B and T cells specific to each pathogen. B cells, upon recognizing the specific antigens, differentiate into plasma cells, producing antibodies that neutralize each of the targeted pathogens. Meanwhile, cytotoxic T cells recognize and destroy infected cells, offering protection against intracellular pathogens such as viruses (Folegatti et al., 2020).
Multi-pathogen vaccines, such as those utilizing viral vectors, can enhance the immune response by delivering antigens more effectively to APCs. This allows for a more sustained immune response and the development of immune memory, which is critical for long-term protection. Advances in mRNA and vector-based vaccine technology further enhance the efficacy of multi-pathogen vaccines by allowing for the precise inclusion of multiple epitopes, ensuring comprehensive immune coverage against a wide array of pathogens (Baden et al., 2020).
3.3 Potential advantages of multi-pathogen vaccines in disease prevention
The development of multi-pathogen vaccines offers several key advantages in the prevention of infectious diseases. First and foremost, these vaccines can streamline immunization schedules by reducing the number of individual vaccinations needed to protect against multiple diseases. This is particularly beneficial in regions with limited healthcare infrastructure, where multiple clinic visits for vaccination may be challenging (Abbas et al., 2019).
Another advantage of multi-pathogen vaccines is the potential to achieve broad immunity across various pathogens, leading to a more comprehensive and coordinated immune response. For instance, combination vaccines, such as those targeting respiratory pathogens, could simultaneously protect against influenza, SARS-CoV-2, and other viral infections, reducing the overall burden of respiratory diseases (Heath et al., 2021). By inducing immunity against multiple pathogens at once, these vaccines can also minimize the occurrence of immune interference, wherein the immune system prioritizes one antigen over another.
Multi-pathogen vaccines also offer cost-efficiency and logistical benefits. Reducing the number of vaccines required leads to lower production costs and simplified vaccine distribution, which is critical during large-scale vaccination campaigns, particularly in pandemic scenarios. Additionally, by limiting the number of injections needed, multi-pathogen vaccines can improve patient compliance and acceptance, leading to higher overall immunization rates and better public health outcomes (Sekuloski et al., 2018).
4 Safety Profiles of Multi-Pathogen Vaccines
The safety profiles of multi-pathogen vaccines are crucial to their successful development and widespread use. Ensuring that these vaccines provide protection against multiple pathogens without causing adverse reactions or compromising the immune response is a fundamental concern for regulatory bodies and public health officials. Multi-pathogen vaccines must demonstrate that they can be administered safely, both in the short term and over extended periods, while maintaining a robust immunogenic response to each targeted pathogen (Hsieh et al., 2021).
4.1 Common safety issues of multi-pathogen vaccines
Common safety issues related to multi-pathogen vaccines generally mirror those of single-pathogen vaccines, but with additional considerations due to the increased complexity. Typical side effects include local reactions at the injection site, such as pain, redness, or swelling, as well as systemic symptoms like fever, headache, and fatigue. These are usually mild and transient, resolving within a few days after vaccination. In clinical trials, multi-pathogen vaccines like the combined COVID-19 vaccines have shown similar safety profiles to single-target vaccines, with most adverse reactions being mild to moderate (Voysey et al., 2020).
However, multi-pathogen vaccines may also introduce additional complexities, such as potential immune interference, where the immune system may respond unevenly to the multiple antigens presented. This could result in a lower immune response to one or more of the pathogens in the vaccine, potentially reducing the overall effectiveness. In some cases, the inclusion of adjuvants (substances that enhance the immune response) in multi-pathogen vaccines may lead to stronger side effects, including more pronounced local or systemic reactions (Polack et al., 2020).
4.2 Issues related to immunogenicity
Immunogenicity, the ability of a vaccine to provoke an immune response, is a critical factor in assessing vaccine safety and efficacy. In the case of multi-pathogen vaccines, balancing the immune response to multiple antigens without inducing immune interference or overloading the immune system is a key challenge. Studies have shown that while most multi-pathogen vaccines effectively generate immune responses to all included antigens, there are concerns that immune responses to some antigens may be less robust than expected (Flacco et al., 2018).
For example, early trials of combination vaccines such as DTP (diphtheria, tetanus, and pertussis) highlighted the possibility of immune interference, where the immune response to one antigen might be suppressed due to the presence of others (Malfertheiner et al., 2018). More recent vaccines, including the multi-antigenic vaccines targeting COVID-19 variants, have managed to mitigate such interference through optimized formulations, but immunogenicity remains a critical aspect of ongoing vaccine development.
To address these challenges, some multi-pathogen vaccines include adjuvants to enhance the overall immune response. However, while adjuvants can boost the vaccine's effectiveness, they may also increase the risk of local and systemic adverse reactions, making it essential to carefully balance immunogenicity and safety (Heath et al., 2021).
4.3 Long-term safety data
Long-term safety data are essential for assessing the potential risks of multi-pathogen vaccines over time, particularly as these vaccines may be given in multiple doses or boosters. Monitoring the occurrence of adverse events and the durability of the immune response is crucial to ensure that these vaccines continue to be safe and effective years after administration. While some long-term safety data for traditional combination vaccines (such as MMR and DTP) are available, newer multi-pathogen vaccines like those for COVID-19 are still in the process of accumulating long-term safety data.
Preliminary data from long-term studies of multi-pathogen vaccines like the mRNA-based COVID-19 vaccines show that serious adverse events are rare, and the vaccines continue to provide protection over time without significant safety concerns (Baden et al., 2020). However, given the relatively recent introduction of many multi-pathogen vaccines, ongoing surveillance and studies are needed to detect any rare, long-term side effects that may arise, such as autoimmune reactions or chronic inflammation.
Furthermore, long-term monitoring is crucial for understanding the vaccine's impact on various population groups, including immunocompromised individuals, pregnant women, and the elderly, who may respond differently to multi-pathogen vaccines. Continued post-marketing surveillance and large-scale epidemiological studies will provide the necessary data to confirm the long-term safety of these vaccines for the broader population (Kelly et al., 2021).
While multi-pathogen vaccines generally demonstrate strong safety profiles, ensuring their long-term safety and efficacy requires continuous monitoring and improvement. With the growing complexity of vaccine formulations and the rapid pace of vaccine development, the challenge of balancing immunogenicity and safety remains central to future vaccine research.
5 Mechanisms of Vaccine Efficacy
The efficacy of multi-pathogen vaccines depends on their ability to stimulate a protective immune response across various pathogens. The immune mechanisms underlying this efficacy involve eliciting immune memory, cross-reactivity between related pathogens, and the ability to maintain long-term protection through booster effects. These factors play a pivotal role in ensuring that multi-pathogen vaccines not only protect against the individual pathogens they target but also offer broad, durable immunity.
5.1 Elicitation of immune memory across multiple pathogens
A fundamental aspect of vaccine efficacy is the induction of long-lasting immune memory, which ensures a rapid and effective immune response upon subsequent exposure to the pathogens. Multi-pathogen vaccines aim to generate immunological memory for each of the included pathogens. This process is mediated by memory B cells and T cells, which are primed during the initial vaccination to recognize and respond to specific antigens from each pathogen (Wang et al., 2022).
The challenge in multi-pathogen vaccines is ensuring that immune memory is developed for all the pathogens included in the formulation without compromising the memory response to any individual pathogen. Multi-pathogen vaccines that use protein subunits or viral vectors have been shown to effectively stimulate memory B cells to produce pathogen-specific antibodies for each targeted disease, ensuring broad immunity (Folegatti et al., 2020). For instance, COVID-19 vaccines using viral vectors have demonstrated robust induction of immune memory against multiple viral proteins, suggesting that multi-pathogen vaccines can successfully generate memory across diverse pathogens (Voysey et al., 2020).
5.2 Cross-reactivity and cross-immunity among related pathogens
Cross-reactivity and cross-immunity refer to the phenomenon where immune responses generated against one pathogen provide some degree of protection against related pathogens due to shared antigens or similar molecular structures (Covián et al., 2019). This mechanism is particularly beneficial in multi-pathogen vaccines, as it allows for broader protection against pathogens beyond those specifically included in the vaccine. For example, cross-reactivity between influenza strains and between coronaviruses can lead to enhanced immunity against emerging variants.
Multi-pathogen vaccines that target families of related pathogens, such as respiratory viruses, have the potential to exploit cross-reactivity for broader protection. Research on multi-pathogen vaccines targeting respiratory pathogens has demonstrated that antibodies produced in response to one virus, such as influenza, may also neutralize other similar viruses like coronaviruses, leading to partial cross-immunity (Heath et al., 2021). This cross-immunity can be particularly advantageous in pandemic scenarios, where rapid mutations in viral strains can reduce the effectiveness of vaccines targeting a single pathogen.
The ability to leverage cross-reactivity in multi-pathogen vaccines enhances their overall efficacy, especially in environments where multiple related pathogens co-circulate. However, ensuring that cross-reactivity does not lead to immune interference, where the immune system favors one pathogen over another, is a critical consideration in vaccine design (Sekuloski et al., 2018).
5.3 Booster effects and long-term protection
Booster doses play an essential role in reinforcing the immune response and maintaining long-term protection against multiple pathogens. For multi-pathogen vaccines, boosters are crucial in sustaining immunity, particularly when immune responses to certain pathogens wane over time. Booster doses re-stimulate memory B and T cells, ensuring that the immune system remains primed to respond effectively to each of the included pathogens.
Evidence from multi-pathogen vaccines like the combination vaccines for diphtheria, tetanus, and pertussis (DTP) has shown that booster doses significantly enhance long-term immunity by increasing antibody titers and maintaining immune memory (Malfertheiner et al., 2018). Similarly, booster doses of the COVID-19 vaccines have been shown to improve immune responses against both the original virus strain and emerging variants, ensuring ongoing protection in the face of evolving pathogens (Baden et al., 2020).
Long-term protection provided by multi-pathogen vaccines is also enhanced by the use of adjuvants, which stimulate stronger and more durable immune responses. These adjuvants, when included in booster formulations, can further extend the duration of protection, making multi-pathogen vaccines an effective tool for managing diseases that require long-term immunity, such as respiratory infections and bacterial diseases.
6 Case Studies of Multi-Pathogen Vaccines
6.1 Case study 1: universal influenza vaccine
Influenza viruses mutate frequently, making the development of a universal influenza vaccine a significant scientific challenge. Traditional flu vaccines need to be reformulated annually based on predictions of which strains will be dominant. A universal influenza vaccine, however, aims to provide broad protection against multiple strains of the virus, potentially reducing the need for yearly vaccination.
Several universal influenza vaccines are currently under development, targeting conserved regions of the influenza virus that are less prone to mutation. One approach includes vaccines that induce cross-reactive immune responses by targeting the hemagglutinin stem, a part of the virus that remains relatively unchanged across different strains. Early-stage clinical trials have shown promising results, with vaccines inducing broad immunity against various influenza subtypes and demonstrating favorable safety profiles (Heath et al., 2021).
The universal influenza vaccine represents a significant leap in multi-pathogen vaccine development, with the potential to drastically reduce the global burden of influenza and offer a model for similar efforts against other rapidly mutating viruses, such as coronaviruses.
6.2 Case study 2: vaccine for recurrent urinary tract infections
Recurrent urinary tract infections (UTIs), often caused by Escherichia coli (E. coli), present a significant clinical challenge, particularly for individuals prone to frequent infections. The development of a multi-pathogen vaccine to prevent recurrent UTIs has focused on targeting the uropathogens responsible for these infections, primarily E. coli and other bacteria like Klebsiella pneumoniae.
Recent advances in UTI vaccine development include subunit vaccines that use components of the bacterial pili, which are responsible for bacterial adhesion to the urinary tract. These vaccines aim to stimulate the production of antibodies that prevent bacteria from attaching to the bladder wall, thus preventing infection. Clinical trials of these vaccines have shown that they can reduce the incidence of recurrent infections in individuals with a history of frequent UTIs, with mild to moderate side effects reported (Sekuloski et al., 2018).
The development of a multi-pathogen UTI vaccine could drastically reduce the use of antibiotics for recurrent infections, helping to combat antibiotic resistance while offering long-term protection against multiple uropathogens.
6.3 Case study 3: porcine reproductive and respiratory syndrome vaccine
Porcine reproductive and respiratory syndrome (PRRS) is a significant viral disease affecting swine populations worldwide, causing reproductive failure in breeding animals and respiratory illness in young pigs. The economic impact of PRRS is substantial, prompting the need for effective vaccines that can control this multi-pathogen disease, which is caused by both European (PRRSV-1) and North American (PRRSV-2) viral strains.
The development of multi-pathogen vaccines targeting both strains of the PRRS virus has been a focus of veterinary vaccine research. Current vaccines employ modified live virus (MLV) technology, offering protection against both strains, though efficacy can vary due to the genetic diversity of the virus. Newer approaches include vaccines that utilize viral vector platforms or inactivated virus formulations to provide broader protection against both PRRSV-1 and PRRSV-2. These vaccines have been shown to reduce viral shedding, improve survival rates, and limit transmission, with safety profiles comparable to single-pathogen vaccines (Malfertheiner et al., 2018).
PRRS vaccines serve as a critical tool for managing this disease, illustrating the importance of multi-pathogen vaccine strategies in animal health. Continued efforts to improve vaccine efficacy and broaden immune protection across viral variants will enhance disease control in swine herds globally.
7 Strategies to Enhance Safety and Efficacy
7.1 Incorporation of adjuvants to enhance immune responses
Adjuvants are substances added to vaccines to improve the strength, quality, and duration of the immune response. The use of adjuvants is especially important in multi-pathogen vaccines, where stimulating an effective immune response against multiple antigens simultaneously can be challenging (Facciolà et al., 2022). Adjuvants like aluminum salts (alum), oil-in-water emulsions (MF59), and newer adjuvant systems such as AS03 and CpG 1018 have been shown to significantly enhance the immunogenicity of vaccines by promoting stronger T-cell and antibody responses (Malfertheiner et al., 2018).
For example, the use of the AS03 adjuvant in the H1N1 influenza vaccine not only increased antibody titers but also improved cross-reactivity with different strains of the virus, offering broader protection. Similar adjuvants are being explored in multi-pathogen vaccines to enhance immune responses while ensuring balanced protection against all pathogens included in the formulation (Heath et al., 2021).
7.2 Optimized vaccine dosing regimens
Vaccine dosing regimens play a crucial role in determining both the efficacy and safety of multi-pathogen vaccines. Optimizing the number of doses and the intervals between them can significantly enhance immune responses and provide long-term protection. Multi-dose regimens often allow the immune system to develop a stronger and more sustained response, as seen with vaccines like the hepatitis B and HPV vaccines, which use a three-dose schedule to maximize immunogenicity (Elizaga et al., 2018).
In multi-pathogen vaccines, carefully spaced doses ensure that the immune system has time to respond to each antigen without becoming overwhelmed. Research into COVID-19 vaccines has shown that a second or even third booster dose can significantly increase the production of neutralizing antibodies and improve long-term immunity, making it a key consideration for other multi-pathogen vaccines as well (Voysey et al., 2020).
7.3 Development of delivery systems
Innovative delivery systems are critical to enhancing the safety and efficacy of multi-pathogen vaccines. These systems are designed to ensure that the vaccine components reach the correct immune cells efficiently and generate a potent immune response. Nanoparticle-based delivery systems, liposomes, and viral vectors are examples of cutting-edge technologies that can improve the stability, targeting, and release profiles of multi-pathogen vaccines.
Nanoparticle delivery systems, for instance, encapsulate antigens in a protective shell that improves their stability and promotes their uptake by antigen-presenting cells (APCs). These systems can also release antigens slowly over time, providing a sustained immune stimulus. Viral vectors, like those used in the COVID-19 vaccines, are engineered to deliver genetic material encoding multiple pathogen antigens directly to the host cells, ensuring a strong and localized immune response (Folegatti et al., 2020).
These advancements in delivery technology allow for more precise immune responses, greater vaccine stability, and the potential for single-dose formulations, which would simplify vaccine administration and improve coverage.
7.4 Use of synergistic agents to mitigate potential side effects and enhance vaccine potency
Synergistic agents can be employed to enhance vaccine potency and reduce potential side effects by modulating the immune response. These agents may include immune modulators, other biologics, or small molecules that work in concert with vaccine antigens to direct the immune response more effectively.
For instance, using immune checkpoint inhibitors as synergistic agents in conjunction with vaccines has shown promise in preclinical models by enhancing the immune system's ability to fight off infections while preventing overactive immune responses that could lead to autoimmunity or excessive inflammation. Additionally, the combination of adjuvants and synergistic agents can help fine-tune the balance between an adequate immune response and the avoidance of excessive side effects such as inflammation or fever (Polack et al., 2020).
Using these agents ensures that vaccines remain highly effective while minimizing adverse effects, making multi-pathogen vaccines safer and more tolerable for a broader range of populations, including vulnerable groups such as children, the elderly, and immunocompromised individuals.
8 Challenges in Multi-Pathogen Vaccine Development
8.1 Technical and manufacturing challenges associated with creating multi-pathogen vaccines
One of the most significant technical challenges in developing multi-pathogen vaccines is formulating a product that maintains immunogenicity for all targeted pathogens. Each pathogen has distinct immunological requirements, and combining multiple antigens into a single vaccine formulation can result in immune interference. Immune interference occurs when the immune system fails to respond equally to all the antigens present, which can lead to suboptimal protection against some of the targeted pathogens. Balancing these immune responses is a complex task that requires extensive preclinical and clinical testing (Malfertheiner et al., 2018).
Manufacturing multi-pathogen vaccines also poses considerable challenges. The inclusion of different antigens in a single vaccine can complicate the production process, especially when these antigens have varying stability, storage, and handling requirements. Some antigens may require specific adjuvants or stabilizers to maintain potency, which can increase the complexity and cost of production. Additionally, scaling up production to meet global demand, especially in pandemic situations, adds another layer of difficulty, as manufacturing facilities must ensure that all components of the vaccine are produced consistently and safely (Heath et al., 2021).
8.2 Regulatory hurdles in approval and safety assessment
The regulatory approval process for multi-pathogen vaccines is more complicated than for single-pathogen vaccines due to the need for thorough evaluation of each component of the vaccine. Regulatory agencies, such as the FDA and EMA, require robust data on the safety, efficacy, and immunogenicity of all included antigens. This means that multi-pathogen vaccines must undergo extensive clinical trials to ensure that they do not compromise the immune response to any individual pathogen (Tafreshi, 2020).
Additionally, safety assessments must account for the potential of adverse effects caused by the interaction of different vaccine components. For instance, combining multiple antigens or using certain adjuvants could increase the risk of adverse reactions, including inflammation or autoimmune responses. Regulatory bodies require extensive data to ensure that the combined vaccine is both safe and effective across diverse populations, including children, the elderly, and immunocompromised individuals. This lengthens the approval timeline, increases the cost of development, and may delay the availability of these vaccines to the public (Polack et al., 2020).
8.3 Public acceptance and logistical challenges in mass distribution
Even after overcoming technical and regulatory hurdles, public acceptance remains a critical factor in the success of multi-pathogen vaccines. Vaccine hesitancy, fueled by concerns over safety, efficacy, or distrust of pharmaceutical companies, can hinder the widespread adoption of multi-pathogen vaccines. Public perception of multi-pathogen vaccines may be even more skeptical due to their complexity, as some people might view a single vaccine designed to protect against multiple diseases as more risky than traditional vaccines that target individual pathogens.
Moreover, logistical challenges in the mass distribution of multi-pathogen vaccines cannot be ignored. These vaccines often require specific storage conditions, such as refrigeration or freezing, which can make distribution difficult, particularly in low- and middle-income countries. Ensuring the stability and effectiveness of multi-pathogen vaccines throughout the distribution chain is critical, and disruptions in cold chain logistics can lead to vaccine degradation and decreased efficacy (Baden et al., 2020).
Large-scale vaccine rollouts require extensive coordination between governments, healthcare providers, and global organizations to ensure equitable access to vaccines. Challenges in infrastructure, healthcare staffing, and funding can hinder the successful mass distribution of multi-pathogen vaccines, especially in resource-limited settings.
While multi-pathogen vaccines hold great promise for public health, their development is hindered by a variety of technical, regulatory, and logistical challenges. Addressing these challenges through advancements in vaccine formulation, streamlined regulatory pathways, and improved distribution systems will be critical for ensuring the success of multi-pathogen vaccines in the global fight against infectious diseases.
9 Future Directions in Multi-Pathogen Vaccine Research
9.1 Advances in synthetic biology and mrna technology
Synthetic biology and mRNA technology are revolutionizing the way vaccines are designed and produced. The rapid success of mRNA vaccines during the COVID-19 pandemic showcased their potential to induce strong immune responses quickly and efficiently. mRNA vaccines work by delivering genetic instructions that enable cells to produce pathogen-specific proteins, which then trigger an immune response. This technology is highly adaptable and can be designed to target multiple pathogens simultaneously, making it ideal for multi-pathogen vaccines.
One of the key advantages of mRNA vaccines is their rapid development and scalability. Scientists can quickly modify mRNA sequences to respond to evolving pathogens, which is crucial for addressing diseases caused by viruses that mutate frequently, such as influenza or coronaviruses (Baden et al., 2020). Furthermore, advances in synthetic biology allow for precise control over the design of vaccine antigens, enhancing immune responses while reducing the risk of immune interference.
In the future, multi-pathogen vaccines may incorporate mRNA platforms that encode multiple antigens from various pathogens in a single dose. This would significantly streamline vaccine production, improve global vaccination efforts, and provide protection against a broader range of diseases. Research into stabilizing mRNA vaccines to improve their storage and distribution, particularly in low-resource settings, is also expected to play a crucial role in their widespread adoption (Heath et al., 2021).
9.2 Exploration of gene editing and CRISPR technology
Gene editing technologies, particularly CRISPR-Cas systems, are opening new avenues in vaccine research. CRISPR technology allows scientists to precisely edit genetic material, which can be applied to the design of more effective vaccines. By using CRISPR to modify pathogen genomes, researchers can create attenuated pathogens for use in live vaccines or modify host immune responses to enhance the effectiveness of vaccines against multiple pathogens.
CRISPR can also be employed to develop vaccines that target genetic markers shared by various pathogens, leading to broader and more efficient immune responses. For instance, the identification of conserved regions of viral genomes using CRISPR-based screening could enable the creation of vaccines that provide cross-protection against a wide array of viral strains and variants, further enhancing the potential of multi-pathogen vaccines (Malfertheiner et al., 2018).
Additionally, CRISPR technology offers potential in improving the safety profile of vaccines by precisely targeting immune modulatory genes to reduce adverse effects. This could make multi-pathogen vaccines more tolerable, particularly for vulnerable populations such as the elderly and immunocompromised individuals. In the future, gene editing may allow for the customization of vaccines based on individual genetic profiles, leading to personalized vaccine strategies.
9.3 Global collaboration
The future success of multi-pathogen vaccine research relies heavily on global collaboration among governments, research institutions, pharmaceutical companies, and international health organizations. The COVID-19 pandemic highlighted the importance of global cooperation in vaccine development, as unprecedented levels of collaboration led to the rapid creation and distribution of multiple vaccines worldwide. This model of cooperation can serve as a blueprint for the development of multi-pathogen vaccines aimed at preventing global pandemics and endemic diseases.
International collaborations can accelerate the sharing of data, technology, and resources, enabling faster vaccine development and approval. Global health organizations, such as the World Health Organization (WHO) and the Coalition for Epidemic Preparedness Innovations (CEPI), have already initiated multi-pathogen vaccine development programs aimed at addressing the risk of future pandemics by preparing vaccines that target multiple pathogens with pandemic potential.
Furthermore, public-private partnerships play a vital role in scaling up the production and distribution of multi-pathogen vaccines, ensuring that they are accessible to all populations, especially in low- and middle-income countries. By fostering collaboration across borders, the scientific community can better prepare for emerging health threats and ensure equitable access to life-saving vaccines on a global scale (Sekuloski et al., 2018).
The future of multi-pathogen vaccine research is bright, with emerging technologies such as synthetic biology, mRNA, and CRISPR transforming the field. Global collaboration will be key to accelerating the development, distribution, and accessibility of these vaccines, ensuring that populations worldwide are protected from a wide range of infectious diseases.
Acknowledgments
I thank the anonymous reviewers for their insightful comments and suggestions for the manuscript.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbas G., Zafar I., Ahmad S., and Azam S.S., 2019, Immunoinformatics design of a novel multi-epitope peptide vaccine to combat multi-drug resistant infections caused by Vibrio vulnificus, European Journal of Pharmaceutical Sciences, 142: 105160.
https://doi.org/10.1016/j.ejps.2019.105160
PMID: 31751777
Adlbrecht C., Wurm R., Depuydt P., Spapen H., Lorente J., Staudinger T., Creteur J., Zauner C., Meier-Hellmann A., Eller P., Laenen M., Molnár Z., Várkonyi I., Schaaf B., Héjja M., Šrámek V., Schneider H., Kanesa-Thasan N., Eder-Lingelbach S., Klingler A., Dubischar K., Wressnigg N., and Rello J., 2020, Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial, Critical Care, 24(1): 74
https://doi.org/10.1186/s13054-020-2778-0
PMID: 32131866 PMCID: PMC7057595
Baden L.R., El Sahly H.M.E., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Kehtan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., and COVE Study Group, 2020, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, The New England Journal of Medicine, 384(5): 403-416.
https://doi.org/10.1056/NEJMoa2035389
PMID: 33378609 PMCID: PMC7787219
Chen S.X., Pounraj S., Sivakumaran N., Kakkanat A., Sam G., Kabir M.T., and Rehm B.H.A., 2023, Precision-engineering of subunit vaccine particles for prevention of infectious diseases, Frontiers in Immunology, 14: 1131057.
https://doi.org/10.3389/fimmu.2023.1131057
PMID: 36817419 PMCID: PMC9935699
Covián C., Fernández-Fierro A., Retamal-Díaz A., Díaz F.E,, Vasquez A.E., Lay M.K., Riedel C.A., González P.A., Bueno S.M., and Kalergis A.M., 2019, BCG-induced cross-protection and development of trained immunity: implication for vaccine design, Frontiers in Immunology, 10: 2806.
https://doi.org/10.3389/fimmu.2019.02806
PMID: 31849980 PMCID: PMC6896902
Elizaga M.L., Li S.Y., Kochar N.K., Wilson G.J., Allen M.A., Tieu H.V.N., Frank I., Sobieszczyk M.E., Cohen K.W., Sanchez B., Latham T.E., Clarke D.K., Egan M., Eldridge J.H., Hannaman D., Xu R., Ota-Setlik A., McElrath M.J., Hay C., and NIAID HIV Vaccine Trials Network (HVTN) 087 Study Team, 2018, Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial, PLoS One, 13(9): e0202753.
https://doi.org/10.1371/journal.pone.0202753
PMID: 30235286 PMCID: PMC6147413
Facciolà A., Visalli G., Laganà A., and Di Pietro A.D., 2022, An overview of vaccine adjuvants: current evidence and future perspectives, Vaccines, 10(5): 819.
https://doi.org/10.3390/vaccines10050819
PMID: 35632575 PMCID: PMC9147349
Fierro C.A., Sarnecki M., Doua J., Spiessens B., Go O., Davies T.A., van de Dobbelsteen G., Poolman J., Abbanat D., and Haazen W., 2023, Safety, reactogenicity, immunogenicity, and dose selection of 10-valent extraintestinal pathogenic Escherichia coli bioconjugate vaccine (VAC52416) in adults aged 60-85 years in a randomized, multicenter, interventional, first-in-human, phase 1/2a study, Open Forum Infectious Diseases, 10(8): ofad417.
https://doi.org/10.1093/ofid/ofad417
PMID: 37608916 PMCID: PMC10442062
Flacco M.E., Manzoli L., Rosso A., Marzuillo C., Bergamini M., Stefanati A., Cultrera R., Villari P., Ricciardi W., Ioannidis J.P.A., and Contopoulos-Ioannidis D.G., 2018, Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: a systematic review and meta-analysis, The Lancet Infectious Diseases, 18(4): 461-472.
https://doi.org/10.1016/S1473-3099(18)30048-3
PMID: 29371070
Folegatti P.M., Ewer K., Aley P., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S.N., Bittaye M., Clutterbuck E., Dold C., Faust S., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., and Oxford COVID Vaccine Trial Group, 2020, Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial, Lancet, 396(10249): 467-478.
https://doi.org/10.1016/S0140-6736(20)31604-4
PMID: 32702298 PMCID: PMC7445431
Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., Chadwick D.R., Clark R., Cosgrove C., Galloway J., Goodman A.L., Heer A., Higham A., Iyengar S., Jamal A., Jeanes C., Kalra P.A., Kyriakidou C., McAuley D.F., Meyrick A., Minassian A.M,, Minton J., Moore P., Munsoor I., Nicholls H., Osanlou O., Packham J., Pretswell C.H., Ramos A.S.F., Saralaya D., Sheridan R.P., Smith R., Soiza RL.., Swift P.A., Thomson E.C., Turner J., Viljoen M.E., Albert G., Cho I., Dubovsky F., Glenn G., Rivers J., Robertson A., Smith K., and Toback S., 2019nCoV-302 Study Group, 2021, Safety and efficacy of NVX-CoV2373 Covid-19 vaccine, The New England Journal of Medicine, 385(13): 1172-1183.
https://doi.org/10.1056/NEJMoa2107659
PMID: 34192426 PMCID: PMC8262625
Hsieh S.M., Liu M.C., Chen Y.H., Lee W.S., Hwang S.J., Cheng S.H., Ko W.C., Hwang K.P., Wang N.C., Lee Y.L., Lin Y.L., Shih S.R., Huang C.G., Liao C.C., Liang J.J., Chang C.S., Chen C., Lien C.E., Tai I.C., and Lin T.Y., 2021, Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan, The Lancet Respiratory Medicine, 9(12): 1396-1406.
https://doi.org/10.1016/S2213-2600(21)00432-7
PMID: 34655522 PMCID: PMC8514195
Jongo S.A., Church L.W.P., Nchama V.U.N.N., Hamad A., Chuquiyauri R., Kassim K.R., Athuman T., Deal A., Natasha K.C., Mtoro A., Mpina M., Nyakarungu E., Bidjimi G.O., Owono M.A., Mayé E.R.M.., Mangue M.E.O., Okomo G.N.N., Pasialo B.E.T., Mandumbi D.M.O., Mikue M.S.A., Mochomuemue F.L., Obono M.O., Besahá J.C.M., Bijeri J.R., Abegue G.M., Veri Y.R., Bela I.T., Chochi F.C., Sánchez J.E.L., Pencelli V., Gayozo G., Nlang J.A.E.M., Schindler T., James E.R., Abebe Y., Lemiale L., Stabler T.C., Murshedkar T., Chen M.C., Schwabe C., Ratsirarson J., Rivas M.R., Ayekaba M.O., Milang D.V.N., Falla C.C., Phiri WP.., García G.A., Maas C.D,, Nlavo B.M., Tanner M., Billingsley P.F., Sim B.K.M., Daubenberger C., Hoffman S.L., Abdulla S., and Richie T.L., 2022, Multi-dose priming regimens of PfSPZ vaccine: safety and efficacy against controlled human malaria infection in equatoguinean adults, The American Journal of Tropical Medicine and Hygiene, 106(4): 1215-1226.
https://doi.org/10.4269/ajtmh.21-0942
PMID: 35130487 PMCID: PMC8991366
Jr Frenck R.W., Ervin J., Chu L., Abbanat D., Spiessens B., Go O., Haazen W., van den Dobbelsteen G., Poolman J., Thoelen S., and de Palacios P.i., 2019, Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial, The Lancet Infectious Diseases, 19(6): 631-640.
https://doi.org/10.1016/S1473-3099(19)30084-1
PMID: 31079947
Kelly H., Sokola B., and Abboud H., 2021, Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients, Journal of Neuroimmunology, 356: 577599-577599.
https://doi.org/10.1016/j.jneuroim.2021.577599
PMID: 34000472 PMCID: PMC8095041
Lacaille‐Dubois M.A., 2019, Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: a review, Phytomedicine, 60: 152905-152905.
https://doi.org/10.1016/j.phymed.2019.152905
PMID: 31182297 PMCID: PMC7127804
Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V., Naroditsky B.S., Gintsburg A.L, .and Gam-COVID-Vac Vaccine Trial Group, 2021, Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia, Lancet (London, England), 397: 671-681.
https://doi.org/10.1016/S0140-6736(21)00234-8
PMID: 33545094 PMCID: PMC7852454
Lynn D.J., Benson S.C., Lynn M.A., and Pulendran B., 2021, Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms, Nature Reviews Immunology, 22(1): 33-46.
https://doi.org/10.1038/s41577-021-00554-7
PMID: 34002068 PMCID: PMC8127454
Malfertheiner P., Selgrad M., Wex T., Romi B., Borgogni E., Spensieri F., Zedda L., Ruggiero P., Pancotto L., Censini S., Palla E., Kanesa-thasan N., Scharschmidt B., Rappuoli R., Graham D.Y., Schiavetti F., and Giudice G., 2018, Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a cag-positive strain: a randomised, placebo-controlled phase 1/2 study, The Lancet Gastroenterology & Hepatology, 3(10): 698-707.
https://doi.org/10.1016/S2468-1253(18)30125-0
PMID: 30042064
Pastural É., McNeil S.A., MacKinnon-Cameron D., Ye L.Y., Langley J.M., Stewart R., Martin L.H., Hurley G.J., Salehi S., Penfound T.A., Halperin S., and Dale J.B., 2019, Safety and immunogenicity of a 30-valent M protein-based group A streptococcal vaccine in healthy adult volunteers: a randomized, controlled phase I study, Vaccine, 38(6): 1384-1392.
https://doi.org/10.1016/j.vaccine.2019.12.005
PMID: 31843270
Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., and C4591001 Clinical Trial Group, 2020, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine,The New England Journal of Medicine, 383(27): 2603-2615.
https://doi.org/10.1056/NEJMoa2034577
PMID: 33301246 PMCID: PMC7745181
Saleh A., Qamar S., Tekin A., Singh R., and Kashyap R., 2021, Vaccine development throughout history, Cureus, 13(7): e16635.
https://doi.org/10.7759/cureus.16635
PMID: 34462676 PMCID: PMC8386248
Sekuloski S., Batzloff M.R., Griffin P., Parsonage W., Elliott S., Hartas J., O’Rourke P., Marquart L., Pandey M., Rubin F.A., Carapetis J., McCarthy J., and Good M.F., 2018, Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial, PLoS ONE, 13(7): e0198658.
https://doi.org/10.1371/journal.pone.0198658
PMID: 29965967 PMCID: PMC6028081
Sharma R., Rajput V., Jamal S., Grover A., and Grover S., 2021, An immunoinformatics approach to design a multi-epitope vaccine against Mycobacterium tuberculosis exploiting secreted exosome proteins, Scientific Reports, 11: 13836.
https://doi.org/10.1038/s41598-021-93266-w
PMID: 34226593 PMCID: PMC8257786
Tafreshi S.Y.H., 2020, Efficacy, safety, and formulation issues of the combined vaccines, Expert Review of Vaccines, 19(10): 949-958.
https://doi.org/10.1080/14760584.2020.1817551
PMID: 33118470
Wang C.Y., Hwang K.P., Kuo H.K., Peng W.J., Shen Y.H., Kuo B.S., Huang J.H., Liu H., Ho Y.H., Lin F., Ding S.D., Liu Z., Wu H.T., Huang C.T., Lee Y.J., Liu M.C., Yang Y.C., Lu P.L., Tsai H.C., Lee C.H., Shi Z.Y., Liu C.E., Liao C.H., Chang F.Y., Cheng H.C., Wang F.D., Hou K.L., Cheng J., Wang M.S., Yang Y.T., Chiu H.C., Jiang M.H., Shih H.Y., Shen H.Y., Chang P.Y., Lan Y.R., Chen C.T., Lin Y.L., Liang J.J., Liao C.C., Chou Y.C., Morris M.K., Hanson C.V., Guirakhoo F., Hellerstein M., Yu H.J., King C.C., Kemp T., Heppner D.G., and Monath T.P., 2022, A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against delta and omicron variants, The Journal of Clinical Investigation, 132(10): e157707.
https://doi.org/10.1172/JCI157707
Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E.M., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., and Oxford COVID Vaccine Trial Group, 2020, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet (London, England), 397(10269): 99-111.
https://doi.org/10.1016/S0140-6736(20)32661-1
PMID: 33306989 PMCID: PMC7723445
Xu L.H., Feng T.T., and Fang K.Y., 2024, Safety and efficacy of mRNA vaccines: insights from clinical trials, International Journal of Clinical Case Reports, 14(3): 117-131.
https://dx.doi.org/10.5376/ijccr.2024.14.0014
Xu W., and Li J.H., 2024, Peptide-based vaccines for oral cancer: mechanisms of action and clinical outcomes, International Journal of Clinical Case Reports, 14(3): 132-143.
https://dx.doi.org/10.5376/ijccr.2024.14.0015
Xu S.Q., Yang K.P., Li R.S., and Zhang L., 2020, mRNA vaccine era—mechanisms, drug platform and clinical prospection, International Journal of Molecular Sciences, 21(18): 6582.
https://doi.org/10.3390/ijms21186582
PMID: 32916818 PMCID: PMC7554980
Zhou F., Hansen L., Pedersen G., Grødeland G., and Cox R., 2021, Matrix M adjuvanted H5N1 vaccine elicits broadly neutralizing antibodies and neuraminidase inhibiting antibodies in humans that correlate with in vivo protection, Frontiers in Immunology, 12: 747774.
https://doi.org/10.3389/fimmu.2021.747774
PMID: 34887855 PMCID: PMC8650010
Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.S., Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D., Deng P., Gou J.B., Wang X.W., Wang X.H., and Chen W., 2020, Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial, The Lancet, 396(10249): 479-488.
https://doi.org/10.1016/S0140-6736(20)31605-6
PMID: 32702299 PMCID: PMC7836858

. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yong Zhang
Related articles
. Multi-pathogen vaccines
. Vaccine safety
. Vaccine efficacy
. Immune interference
. mRNA technology
Tools
. Post a comment